Draw The Electrondot Formula For Sicl2Br2
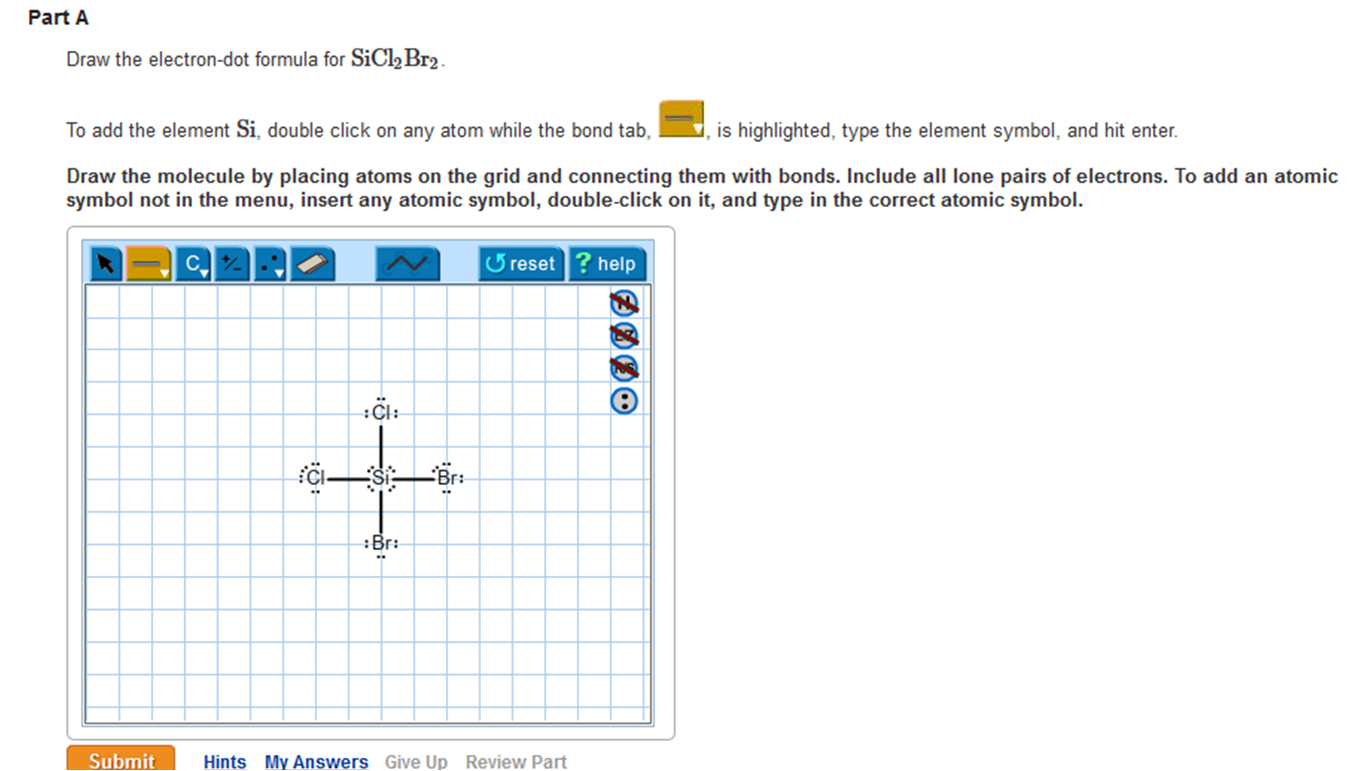
Draw The Electrondot Formula For Sicl2Br2 - Web total valence electrons in sicl2br2 molecule = valence electrons given by 1 silicon atom + valence electrons given by 2 chlorine atoms + valence electrons given by 2 bromine atoms = 4 + 7 (2) + 7 (2) = 32. Web lewis structures are very similar to electron dot diagrams except for the fact that shared electrons between atoms are shown as lines. (valence electrons are the number of electrons present in the outermost shell of an atom). Web draw the lewis structure for sicl2br2. Lone pairs of electrons themselves are usually represented by dots around the atoms. Web this video shows you how to draw the lewis dot structure for sicl2br2. To add the element si, either double click on any atom and type the element symbol, or access a periodic table of elements from the more draw the molecule by placing atoms on the grid and connecting them with bonds. The atom with the least electronegative value is placed at the center. Web it is calculated using the formula given below. The number of dots equals the number of valence electrons in the atom. Lone pairs of electrons themselves are usually represented by dots around the atoms. Include all lone pairs of electrons. It also provides the molecular geometry and bond angle for sicl2br2 as well. To add the element si, either double click on any atom and type the element symbol, or access a periodic table of elements from the more draw the. Web total valence electrons in sicl2br2 molecule = valence electrons given by 1 silicon atom + valence electrons given by 2 chlorine atoms + valence electrons given by 2 bromine atoms = 4 + 7 (2) + 7 (2) = 32. Web how do you draw a lewis structure for sicl_2br_2? Draw the molecule by placing atoms on the grid. Become a study.com member to unlock this answer! 22k views 10 years ago. In the typical lecture, you end up with the final formula but you may not remember all of the individual steps that were done in drawing the formula. Web the energy of the electrostatic attraction ( e e ), a measure of the force’s strength, is inversely. The atom with the least electronegative value is placed at the center. Here’s the best way to solve it. Now decide the central atom. Also, helium is shown in group 8a, but it only has two valence electrons. E = kq1q2 r (12.1.5) (12.1.5) e = k q 1 q 2 r. In order to draw the lewis structure of sicl2br2, first of all you have to find the total number of valence electrons present in the sicl2br2 molecule. These diagrams are helpful because they allow us to predict the shape of the molecule and see the positions of each atom. (valence electrons are the number of electrons present in the outermost. Web the energy of the electrostatic attraction ( e e ), a measure of the force’s strength, is inversely proportional to the internuclear distance between the charged particles ( r r ): E = kq1q2 r (12.1.5) (12.1.5) e = k q 1 q 2 r. Web total valence electrons in sicl2br2 molecule = valence electrons given by 1 silicon. In the typical lecture, you end up with the final formula but you may not remember all of the individual steps that were done in drawing the formula. Web it is calculated using the formula given below. To add the element si, double click on any atom while the bond tab, is highlighted, type the element symbol, and hit enter.. Web it is calculated using the formula given below. Web draw the lewis structure for sicl2br2. Draw the molecule by placing atoms on the grid and connecting them with bonds. It also provides the molecular geometry and bond angle for sicl2br2 as well. In order to draw the lewis structure of sicl2br2, first of all you have to find the. This is done by adding the valence shell electrons of all the constituent atoms. Here’s the best way to solve it. Steps to draw the lewis structure of sicl2br2. Web total valence electrons in sicl2br2 molecule = valence electrons given by 1 silicon atom + valence electrons given by 2 chlorine atoms + valence electrons given by 2 bromine atoms. Web this video shows you how to draw the lewis dot structure for sicl2br2. This problem has been solved! Become a study.com member to unlock this answer! Where each ion’s charge is represented by the symbol q. 22k views 10 years ago. The number of dots equals the number of valence electrons in the atom. To properly draw the sicl 2 br 2 lewis structure, follow these steps: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw the molecule by placing atoms on the grid and connecting them with bonds. The atom with the least electronegative value is placed at the center. To add the element si, double click on any atom while the bond tab, is highlighted, type the element symbol, and hit enter. This problem has been solved! Part a draw the lewis structure for sicl,br2. Draw the molecule by placing atoms on the grid and connecting them with bonds. Web so the total number of valence electrons = valence electrons of silicon atom + (valence electrons of chlorine atom × 2) + (valence electrons of bromine atom × 2) therefore, the total number of valence electrons = 4 + 14 + 14 = 32. In order to draw the lewis structure of sicl2br2, first of all you have to find the total number of valence electrons present in the sicl2br2 molecule. In the typical lecture, you end up with the final formula but you may not remember all of the individual steps that were done in drawing the formula. #2 next, indicate lone pairs on the atoms. Draw the molecule by placing atoms on the grid and connecting them with bonds. #2 next, indicate lone pairs on the atoms. Steps to draw the lewis structure of sicl2br2.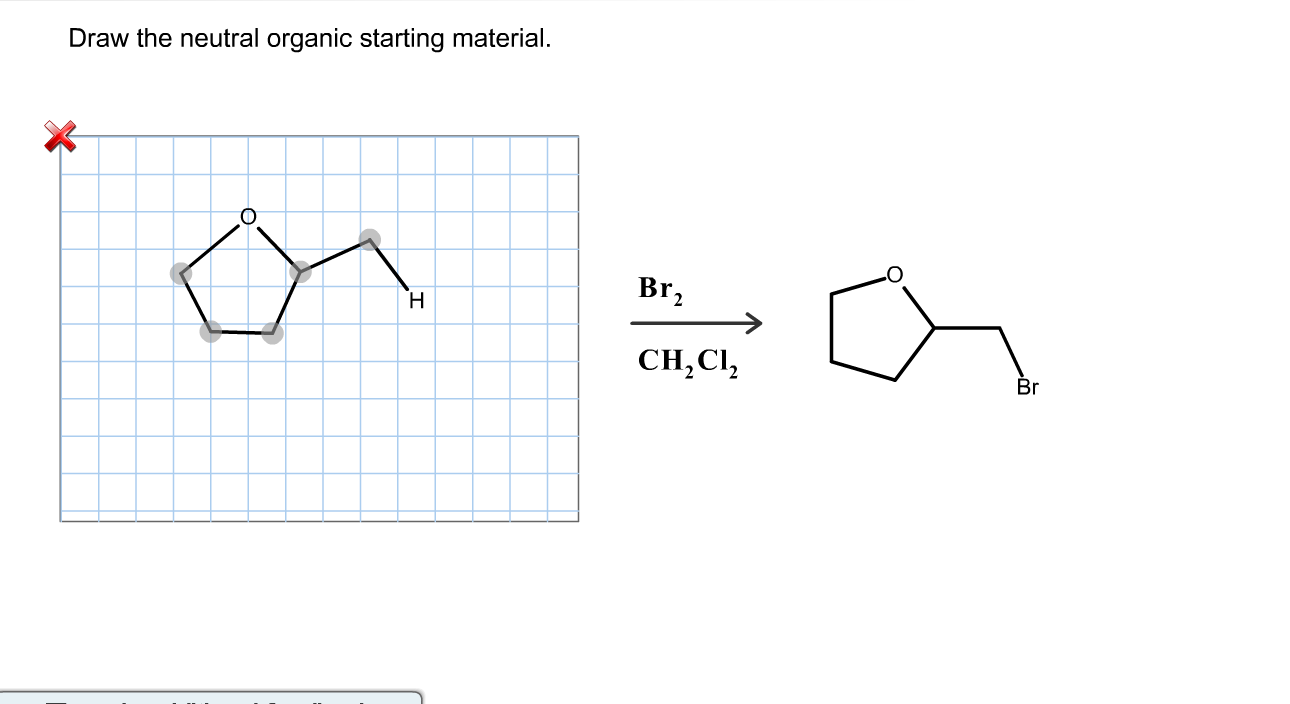
lewis structure for sicl2br2
Solved Draw the electrondot formula for SiCl2Br2. This is
lewis structure for sicl2br2
Lewis Dot Structure For Sicl2br2 alter playground

SiCl2Br2 Lewis Structure How to Draw the Lewis Structure for SiCl2Br2
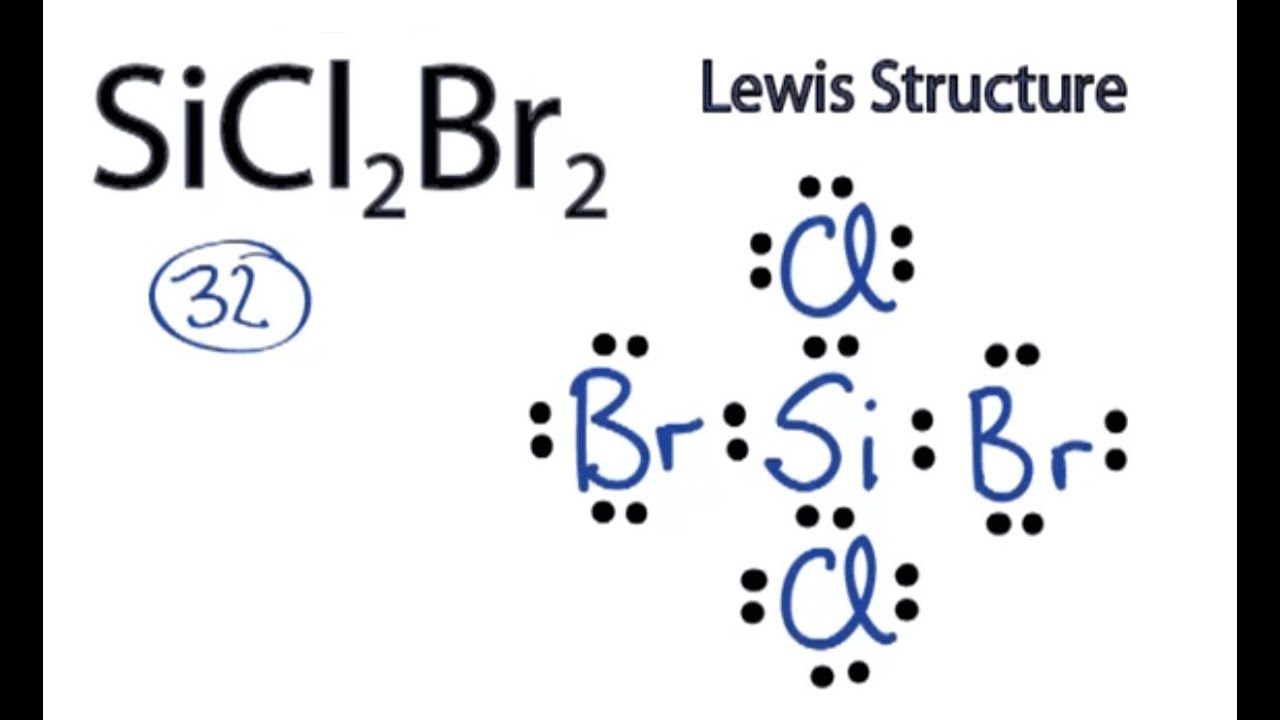
SiCl2Br2 Lewis Structure How to Draw the Lewis Structure for SiCl2Br2
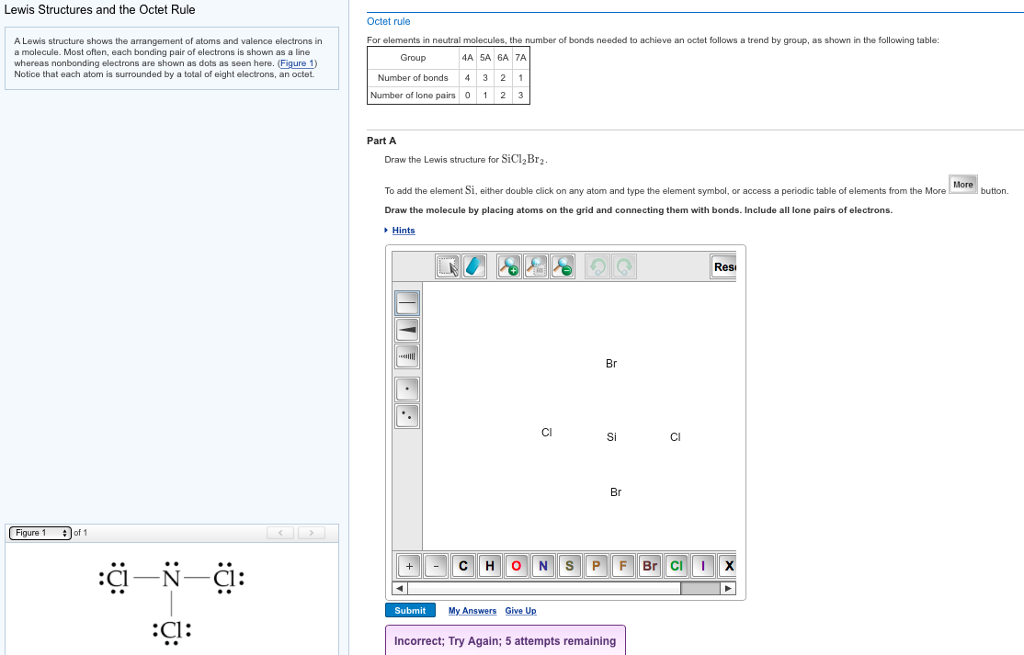
Draw The Lewis Structure For Sicl2br2

SiCl2Br2 Lewis Structure, Geometry, Hybridization, and Polarity
Solved Draw the electrondot formula for SiCl2Br2. To add
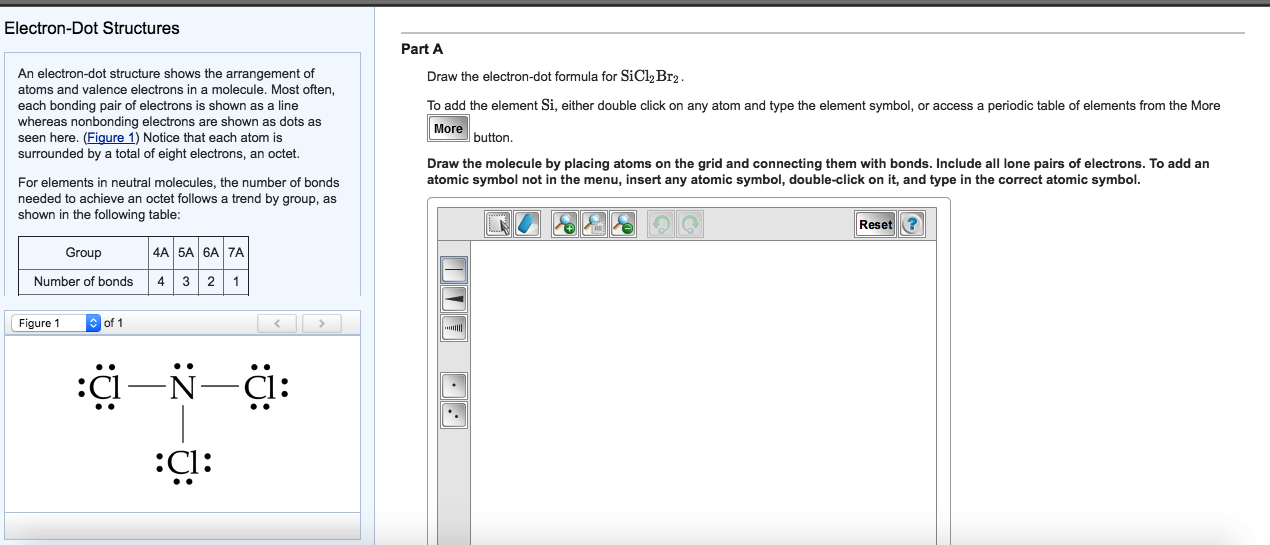
lewis structure for sicl2br2
To Add The Element Si, Either Double Click On Any Atom And Type The Element Symbol, Or Access A Periodic Table Of Elements From The More Draw The Molecule By Placing Atoms On The Grid And Connecting Them With Bonds.
To Add The Element Si, Either Double Click On Any Atom And Type The Element Symbol, Or Access A Periodic Table Of Elements From The More Button.
This Is Done By Adding The Valence Shell Electrons Of All The Constituent Atoms.
#1 Draw A Rough Sketch Of The Structure.
Related Post: