Ammonia Drawing
Ammonia Drawing - It is clear to understand that the geometrical structure of nh3 will be bent. Drawing the lewis structure for nh3. Nitrogen (n) is in group 15 of the periodic table, which means it has 5 valence electrons. Reference range (s) ≤72 umol/l. Why the test is performed. This will help you understand the molecule’s electronic structure and bonding. This inorganic compound has a pungent smell. Ammonia is a chemical byproduct of digestion and other bodily processes. Ammonia blood test, nh3 test, serum ammonia test. Steps of drawing the lewis structure of nh3 is explained in detail in this tutorial. Web when the needle is inserted to draw blood, some people feel moderate pain. Date and time specimen was drawn must be written on tube of blood and request form. Valence electrons are the outermost electrons of an atom and are involved in bonding. The nh 3 chemical name is. Web ammonia (umol/l) 0 to 14 days: Others feel only a prick or stinging. Web ammonia (umol/l) 0 to 14 days: It's not particularly difficult but is an important structure. Calculate the total number of valence electrons. There are 8 valence electrons available for the lewis structure for nh 3. Ammonia is a colorless gas with a chemical formula nh 3. Valence electrons are the outermost electrons of an atom and are involved in bonding. This will help you understand the molecule’s electronic structure and bonding. Others feel only a prick or stinging. To begin drawing the nh3 lewis structure, start by counting the total number of valence electrons. Remember, too, that hydrogen only needs two valence electrons to. Web nh 3 (ammonia) is a commonly tested lewis structure. This is a clip from the complete video:. Why the test is performed. A lewis structure is a way to show how atoms share electrons when they form a molecule. It is clear to understand that the geometrical structure of nh3 will be bent. (ax 3 e 1 ). Web the first step in drawing the electron dot formula for ammonia is to determine the number of bonding electrons for each of the atoms. Web how to draw an ammonia atom. Bacteria in your gut and in your cells create. Print share include loinc® in print. Valence electrons are the outermost electrons of an atom and are involved in bonding. To begin drawing the nh3 lewis structure, start by counting the total number of valence electrons. Web a video explanation of how to draw the lewis dot structure for ammonia, along with information about the compound including formal charges, polarity,. It’s a simple blood test that lets your doctor measure how much ammonia is in your blood. Web 6 steps to draw the lewis structure of nh3. Lewis structure of nh3 can be drawn by using valence electrons of nitrogen and hydrogen atoms. Web 3 min read. Here, the given molecule is nh3 (ammonia). It also is a good example of a molecule with a trigonal prymidal molecular geometry. Craig beals shows how to draw the lewis structure for ammonia. Web last modified on mar 18, 2022. Count the total number of valence electrons. It is clear to understand that the geometrical structure of nh3 will be bent. Web draw the lewis diagram as below: This is a clip from the complete video:. Afterward, there may be some throbbing or a slight bruise. Drawing the lewis structure for nh3. A lewis structure is a way to show how atoms share electrons when they form a molecule. Web this chemistry video tutorial explains how to draw the lewis structure of nh3 also known as ammonia.how to draw lewis structures: Web the first step in drawing the electron dot formula for ammonia is to determine the number of bonding electrons for each of the atoms. It’s a simple blood test that lets your doctor measure how much ammonia. Ammonia blood test, nh3 test, serum ammonia test. In the nh 3 lewis structure (and all structures), hydrogen goes on the outside. 498k views 10 years ago lewis structures practice problems with answers. In order to draw the lewis structure of nh3, first of all you have to find the total number of valence electrons present in the nh3 molecule. To begin drawing the nh3 lewis structure, start by counting the total number of valence electrons. This will help you understand the molecule’s electronic structure and bonding. This inorganic compound has a pungent smell. The billing party has sole responsibility for cpt coding. Ammonia is a chemical byproduct of digestion and other bodily processes. Valence electrons are the outermost electrons of an atom and are involved in bonding. Reference range (s) ≤72 umol/l. Ammonia atoms are one nitrogen and three hydrogens. Ammonia is a colorless gas with a chemical formula nh 3. Why the test is performed. Web draw the lewis diagram as below: Craig beals shows how to draw the lewis structure for ammonia.
Pictogram Van Ammonia Molecule Stock Illustratie Illustration of
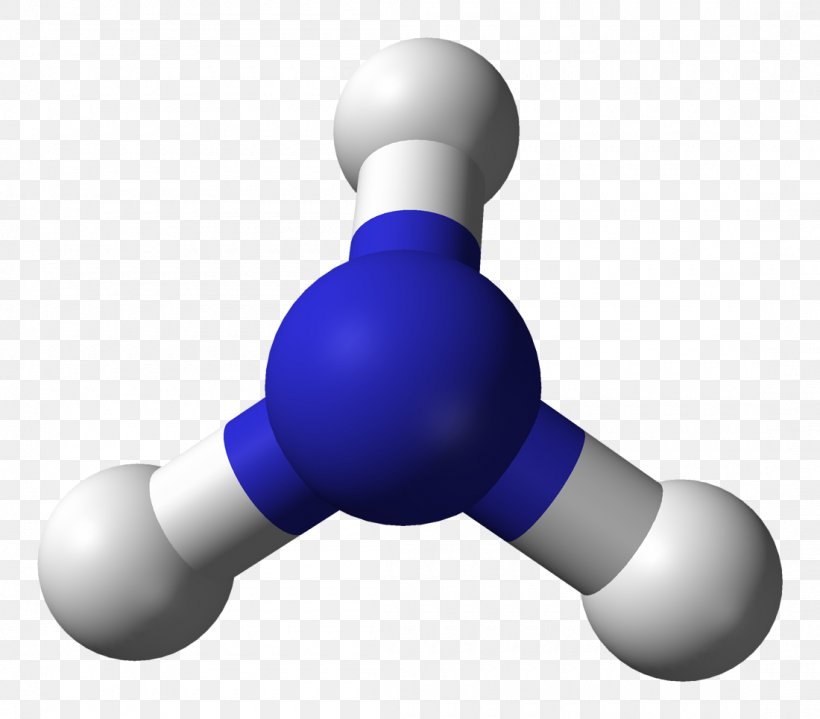
Ammonia Molecule Molecular Geometry Ballandstick Model Lewis

Ammonia Formula
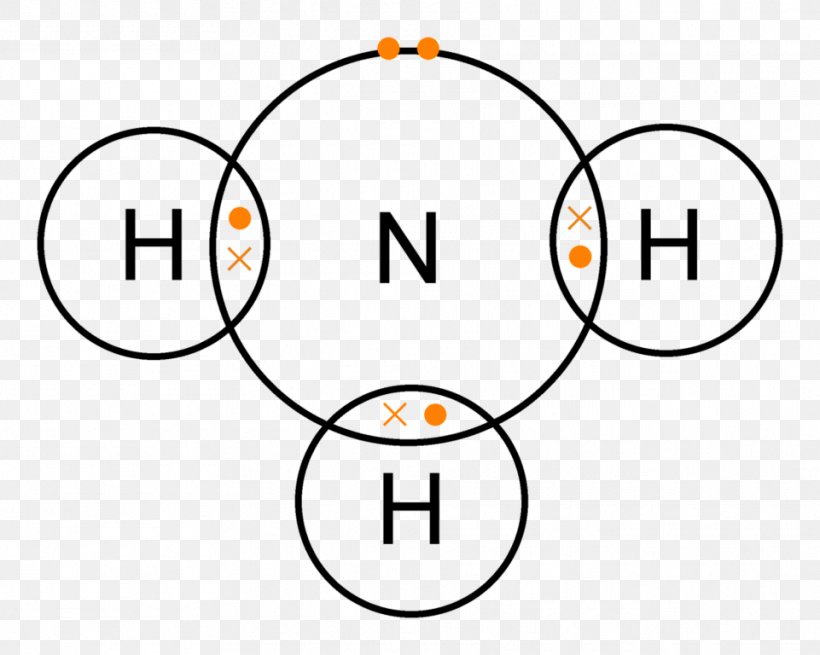
Lewis Structure Ammonia Covalent Bond Lone Pair Chemical Bond, PNG
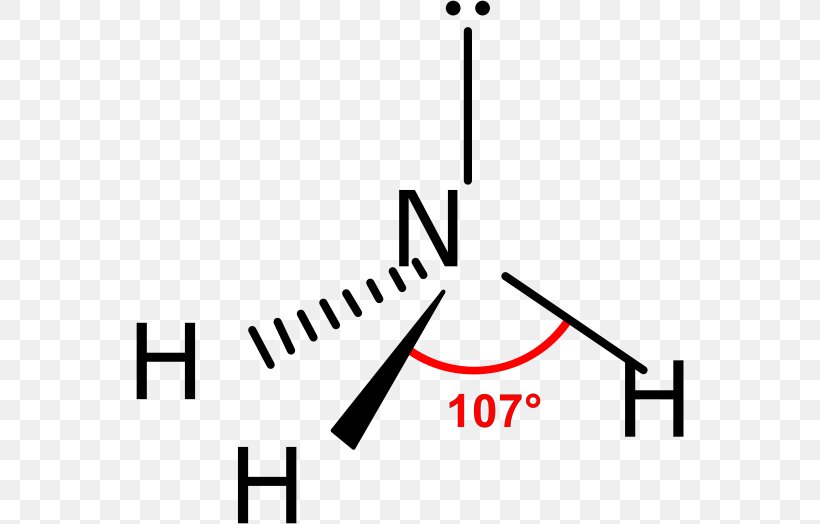
Ammonia Chemical Bond Molecule VSEPR Theory Trigonal Pyramidal

Ammonia molecule 3d structure Royalty Free Vector Image

Ammonia molecule, illustration Stock Image F030/7878 Science
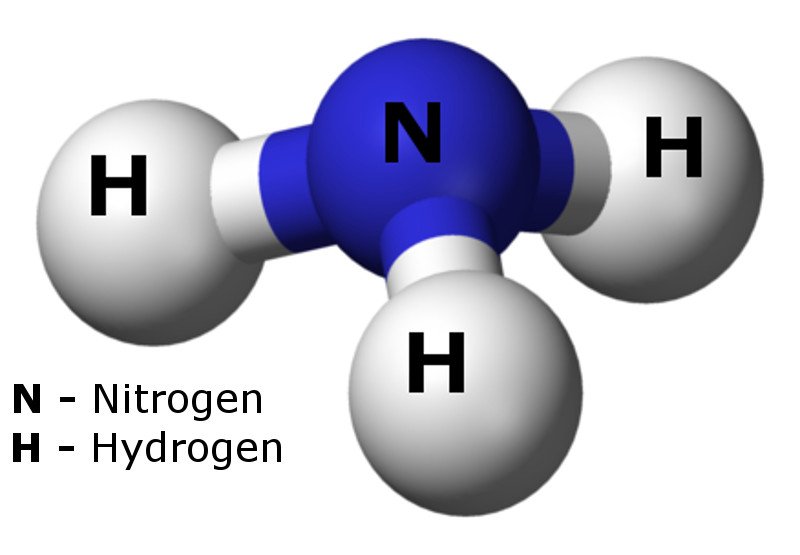
Ammonia Geoviridien
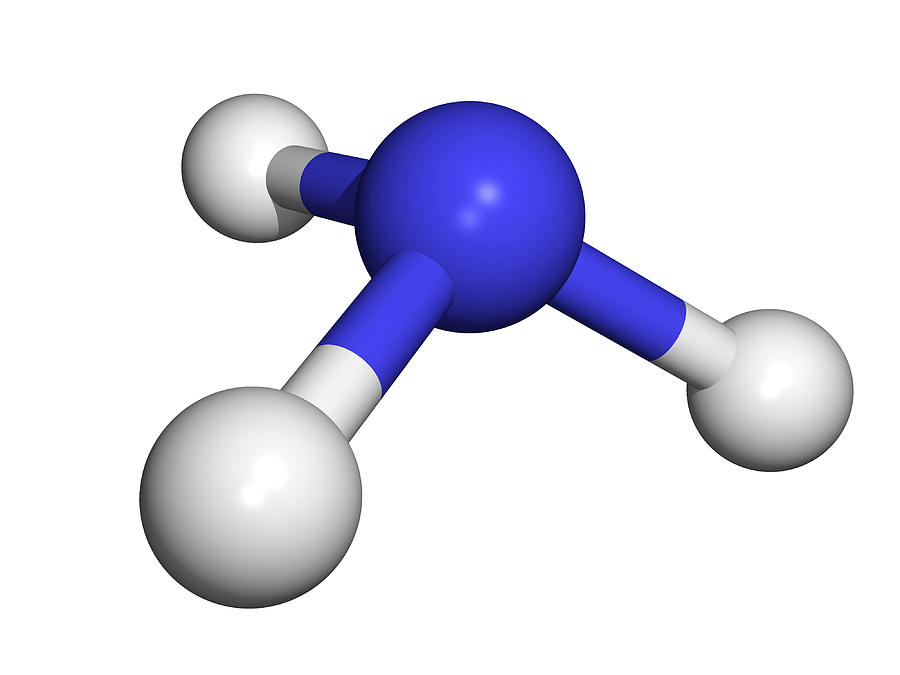
Structure Of Ammonia Molecule

3d render of molecular structure of ammonia isolated over white
A Lewis Structure Is A Way To Show How Atoms Share Electrons When They Form A Molecule.
Web This Chemistry Video Tutorial Explains How To Draw The Lewis Structure Of Nh3 Also Known As Ammonia.how To Draw Lewis Structures:
It’s A Simple Blood Test That Lets Your Doctor Measure How Much Ammonia Is In Your Blood.
Here, The Given Molecule Is Nh3 (Ammonia).
Related Post: