Sulfur Drawing
Sulfur Drawing - Web walkthrough of drawing lewis dot structures. There is a total of 6 electron pairs around the central s atom. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Orbital is the region of space around the nucleus of an atom where electrons are found. To create the lewis structure, we need to determine the total number of valence electrons in sf2. The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. Web choose from 598 sulfur drawings stock illustrations from istock. Calculate the total number of valence electrons. Sulfur (s) = 6 valence electrons. Because there's one sulfur and three oxygen atoms, it is safe to assume that the sulfur is the central atom, surrounded by the three oxygens. Web sulfur fires in the sulfur tank should they occur. Find more chemistry widgets in wolfram|alpha. To write the orbital diagram for the sulfur atom (s) first we need to write the electron configuration for just s. Electrons are the negatively charged particles that orbit the nucleus of an atom. The sulfur trioxide is a tetra atomic chemical molecule where. Because there's one sulfur and three oxygen atoms, it is safe to assume that the sulfur is the central atom, surrounded by the three oxygens. In a formal test, like ap chemistry, which would be considered correct? Calculate the total number of valence electrons. Contents [ hide] sulfur dichloride lewis structure. Write protons, neutrons, and electrons of sulfur atom. Orbital is the region of space around the nucleus of an atom where electrons are found. Web drawing the lewis structure of sf2. Contents [ hide] sulfur dichloride lewis structure. The sulfur (s) atom lies at the center of the structure. 1) determine how the atoms connect to each other. Fluorine (f) = 7 valence electrons each (2 atoms) The nucleus of a sulfur atom contains 16 protons and 16 neutrons. Web elemental sulfur is a bright yellow, crystalline solid at room temperature. Send feedback | visit wolfram|alpha. Direct combustion of sulfur is the only method for producing so 2 (g). The aufbau principle, pauli exclusion principle, and hund’s rule. Calculate the total number of valence electrons. Because there's one sulfur and three oxygen atoms, it is safe to assume that the sulfur is the central atom, surrounded by the three oxygens. Web drawing the lewis structure of sf2. Web steps to draw the bohr model of sulfur atom. This diagram shows how the electrons in the sulfur atom are arranged in different orbitals. Web steps to draw the bohr model of sulfur atom. Sulfur has the atomic number 16 and is denoted by the chemical symbol s. Web this widget gets the lewis structure of chemical compounds. Search by image or video. Sulfur is the tenth most abundant element by mass in the universe and the fifth most abundant on earth. Contents [ hide] sulfur dichloride lewis structure. Want to join the conversation? Web the sulfur orbital diagram is a graphical representation of the electron configuration of the sulfur atom. It is the fifth most abundant molecule on earth’s surface and occurs. Web draw a diagram that summarizes the allotropy of sulfur. Write protons, neutrons, and electrons of sulfur atom. 35k views 4 years ago. Web the sulfur orbital diagram is a graphical representation of the electron configuration of the sulfur atom. Web lewis structure of so3. Web drawing the lewis structure of sf2. Sulfur (s) = 6 valence electrons. Draw nucleus of sulfur atom. Sulfur has 16 protons, 16 neutrons, and 16 electrons. Web sulfuric acid lewis structure: Determine the lewis dot structure for so32− (meaning there are two extra valence electrons in this molecule). The nucleus of a sulfur atom contains 16 protons and 16 neutrons. Sulfur has 16 protons, 16 neutrons, and 16 electrons. Sulfur (s) = 6 valence electrons. Web elemental sulfur is a bright yellow, crystalline solid at room temperature. Draw nucleus of sulfur atom. Contents [ hide] sulfur dichloride lewis structure. Find more chemistry widgets in wolfram|alpha. Web sulfuric acid lewis structure: Web steps to draw the bohr model of sulfur atom. Determine the lewis dot structure for so32− (meaning there are two extra valence electrons in this molecule). The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. The nucleus of a sulfur atom contains 16 protons and 16 neutrons. Direct combustion of sulfur is the only method for producing so 2 (g). Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei. Web choose from 598 sulfur drawings stock illustrations from istock. Sulfur (s) = 6 valence electrons. Web to draw the lewis structure of sulfur dichloride (scl2), place the sulfur atom in the center, connect it with single bonds to two chlorine atoms, and complete the octets by placing lone pairs around each atom. Fluorine (f) = 7 valence electrons each (2 atoms) Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web an electron dot structure for sulfur, which contains both paired and unpaired electrons, is shown below in figure 3.15.1 3.15.:max_bytes(150000):strip_icc()/sulfuratom-58b602563df78cdcd83d5a9d.jpg)
Atom Diagrams Electron Configurations of the Elements
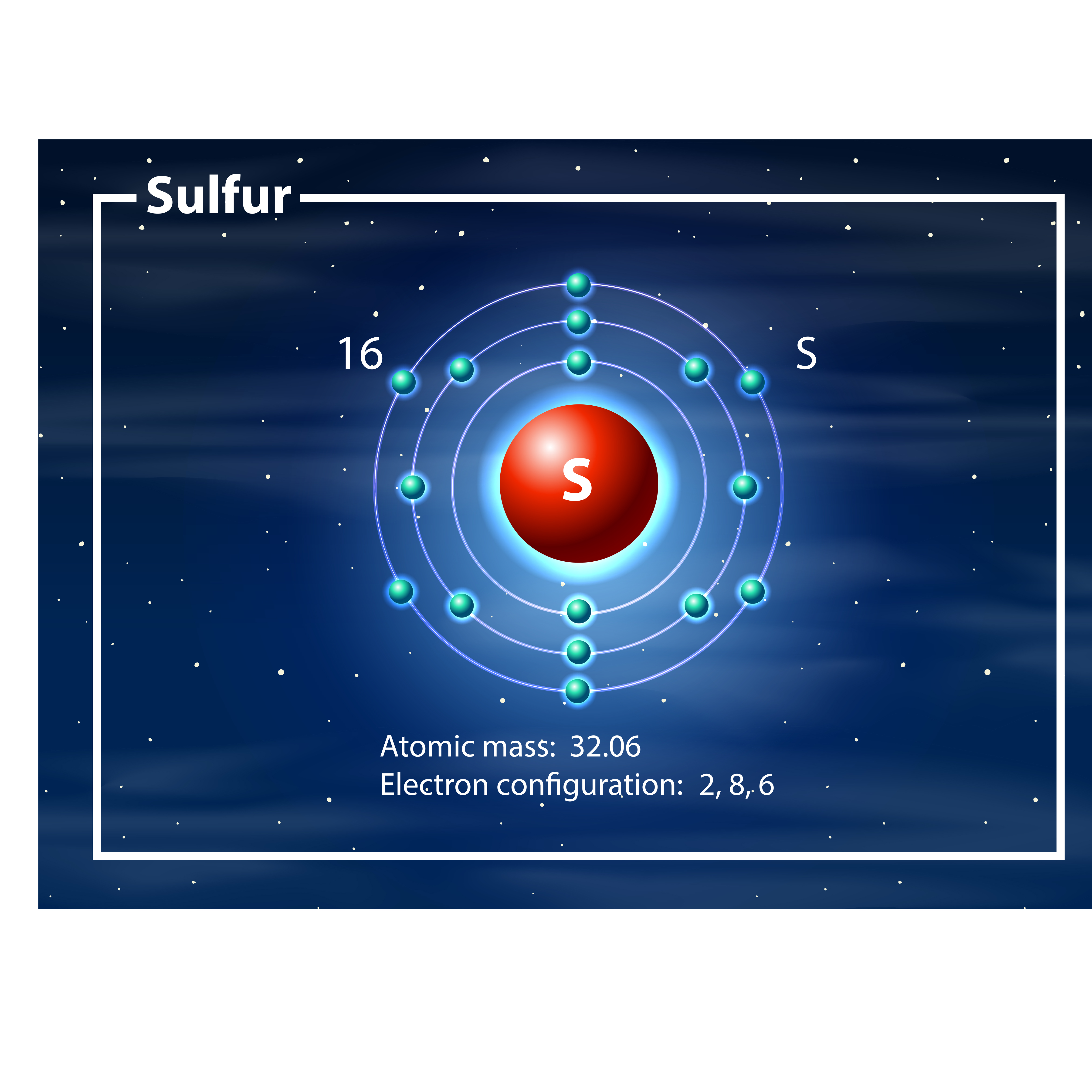
Chemist atom of sulfur diagram 528624 Vector Art at Vecteezy
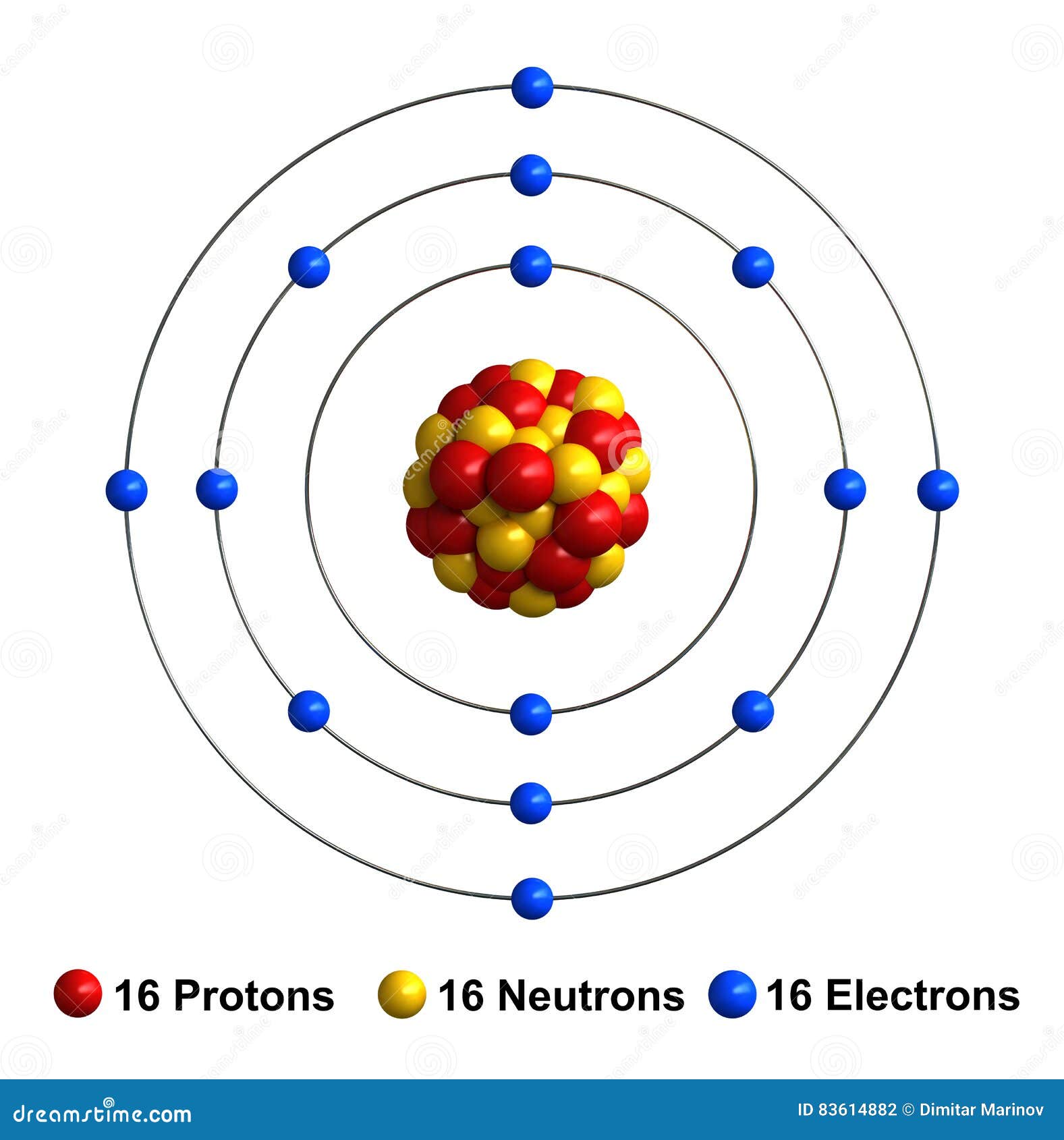
Chemistry 3. Draw the atomic structure of a sulfur atom and a sulfide
Sulfur (S) AMERICAN ELEMENTS

Sulfur Definition, Facts, Symbol, Allotropes, Properties, Uses
O Level Chemistry Sulfur
Sulfur Stock Illustrations 1,549 Sulfur Stock Illustrations, Vectors

Diagram representation of the element sulfur Vector Image

Sulfur symbol. Chemical element of the periodic table. Vector

Sulfur Bohr Model — Diagram, Steps to Draw Techiescientist
Because There's One Sulfur And Three Oxygen Atoms, It Is Safe To Assume That The Sulfur Is The Central Atom, Surrounded By The Three Oxygens.
In This Article, “Sulfuric Acid Lewis Structure”, Drawing The Lewis Structure Of Sulfuric Acid With Detailed Explanations Are Discussed Below.
Loading Ejectors Are Installed At The Sulfur Loading Stations To Prevent Harmful Vapors From Being
Electrons Are The Negatively Charged Particles That Orbit The Nucleus Of An Atom.
Related Post: