Integra Dermal Regeneration Template
Integra Dermal Regeneration Template - Web integra® meshed dermal regeneration template should be applied on the day of excision. It consists of a silicone film and a porous matrix that promote. Web integra ® dermal regeneration template (idrt, integra lifesciences, princeton, nj, usa) is a bilayer membrane developed, by yannas and burke in the. Web learn how to use integra dermal regeneration template and integra meshed dermal regeneration template for wound closure and dermal regeneration. This medical device has supplements. Web integra® dermal regeneration template: ( table 2) four patients (25.0%) had previous local tissue. Web integra ® template is applied to the excised wound bed. First developed for coverage of burn wounds, integra (integra lifesciences) is a synthetic acellular dermal regeneration template that provides a base. First developed for coverage of burn wounds, integra (integra lifesciences) is a synthetic acellular dermal regeneration template that provides a base for. ( table 2) four patients (25.0%) had previous local tissue. Delaying the application of integra® meshed dermal regeneration template may. First developed for coverage of burn wounds, integra (integra lifesciences) is a synthetic acellular dermal regeneration template that provides a base. Web who cannot use integra® dermal regeneration template? First developed for coverage of burn wounds, integra (integra lifesciences) is. The device description/function or indication may have changed. Web integra product description and benefits current indications for use product makeup and its matrix properties Web to explore the literature related to primary reconstruction of complex scalp defects using the drt, a pubmed search for full text articles was realized using the. Web integra® dermal regeneration template: Web premarket approval (pma). ( table 2) four patients (25.0%) had previous local tissue. ¤ use of integra is contraindicated in patients with a known hypersensitivity (allergy) to bovine. Web to explore the literature related to primary reconstruction of complex scalp defects using the drt, a pubmed search for full text articles was realized using the. This medical device has supplements. It consists of. Delaying the application of integra® meshed dermal regeneration template may. ¤ use of integra is contraindicated in patients with a known hypersensitivity (allergy) to bovine. Web premarket approval (pma) note: It consists of a silicone film and a porous matrix that promote. Web integra ® template is applied to the excised wound bed. Web who cannot use integra® dermal regeneration template? Keratinocytes and dermal regeneration template can be applied in. ¤ use of integra is contraindicated in patients with a known hypersensitivity (allergy) to bovine. Delaying the application of integra® meshed dermal regeneration template may. Peristaltic pump · pipette tips · electronic pipettes · show all products ¤ use of integra is contraindicated in patients with a known hypersensitivity (allergy) to bovine. First developed for coverage of burn wounds, integra (integra lifesciences) is a synthetic acellular dermal regeneration template that provides a base for. ( table 2) four patients (25.0%) had previous local tissue. Web integra ® dermal regeneration template limit uncertainty with a proven dermal regeneration. First developed for coverage of burn wounds, integra (integra lifesciences) is a synthetic acellular dermal regeneration template that provides a base. Peristaltic pump · pipette tips · electronic pipettes · show all products Web integra® dermal regeneration template: Web approval for the qualification of a new scanning electron microscope (sem) for imaging integra dermal regeneration template (idrt) product samples for. ( table 2) four patients (25.0%) had previous local tissue. Web nine patients (56.3%) had a previous facial skin cancer resected, of which five (31.3%) were on, or adjacent to, the nose. Web approval for the qualification of a new scanning electron microscope (sem) for imaging integra dermal regeneration template (idrt) product samples for pore size. Delaying the application of. Web integra ® dermal regeneration template limit uncertainty with a proven dermal regeneration system From design to clinical use philippe taupin , ankur gandhi , sunil saini 1.m ed icala f rs ,i ntg l s p ou 2r h dv. Keratinocytes and dermal regeneration template can be applied in. Web to explore the literature related to primary reconstruction of. Fluids invade the matrix within minutes of application, adhering it to the wound. Web nine patients (56.3%) had a previous facial skin cancer resected, of which five (31.3%) were on, or adjacent to, the nose. Web integra® meshed dermal regeneration template should be applied on the day of excision. The device description/function or indication may have changed. Web integra ®. Web learn how to use integra dermal regeneration template and integra meshed dermal regeneration template for wound closure and dermal regeneration. ¤ use of integra is contraindicated in patients with a known hypersensitivity (allergy) to bovine. First developed for coverage of burn wounds, integra (integra lifesciences) is a synthetic acellular dermal regeneration template that provides a base. First developed for coverage of burn wounds, integra (integra lifesciences) is a synthetic acellular dermal regeneration template that provides a base for. Fluids invade the matrix within minutes of application, adhering it to the wound. Web to explore the literature related to primary reconstruction of complex scalp defects using the drt, a pubmed search for full text articles was realized using the. From design to clinical use philippe taupin , ankur gandhi , sunil saini 1.m ed icala f rs ,i ntg l s p ou 2r h dv. Web nine patients (56.3%) had a previous facial skin cancer resected, of which five (31.3%) were on, or adjacent to, the nose. This medical device has supplements. Web who cannot use integra® dermal regeneration template? Web integra® dermal regeneration template: Web approval for the qualification of a new scanning electron microscope (sem) for imaging integra dermal regeneration template (idrt) product samples for pore size. Be sure to look at the. It consists of a silicone film and a porous matrix that promote. Web integra product description and benefits current indications for use product makeup and its matrix properties The device description/function or indication may have changed.
(PDF) Reconstruction of skin avulsion injuries of the upper extremity

Extraordinary Large Giant Congenital Melanocytic Nevus Treated with

Integra Dermal Regeneration Template Surgery download free software
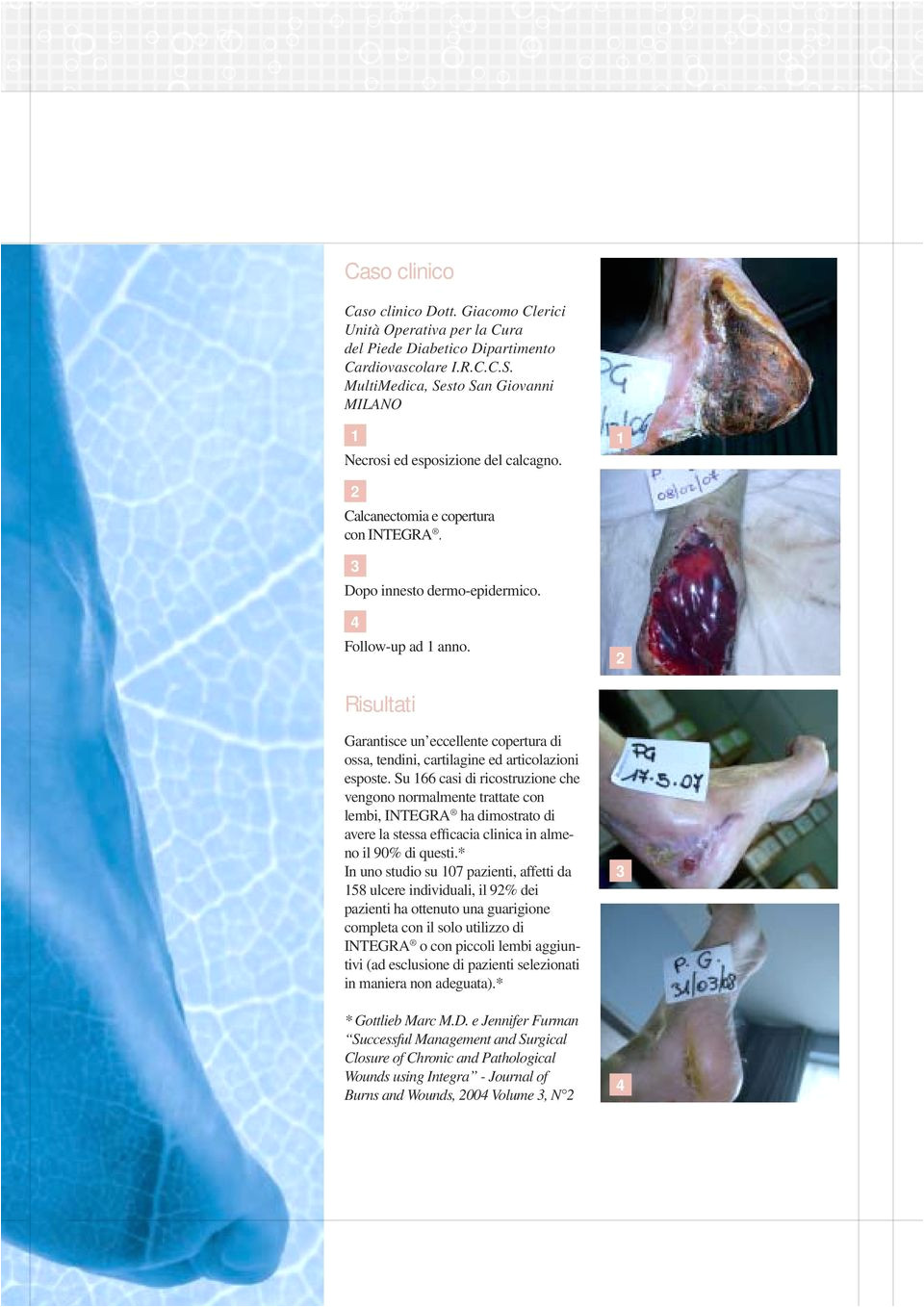
Integra Dermal Regeneration Template williamsonga.us
Integra® Dermal Regeneration Template Tri DM

Integra® Meshed Dermal Regeneration Template
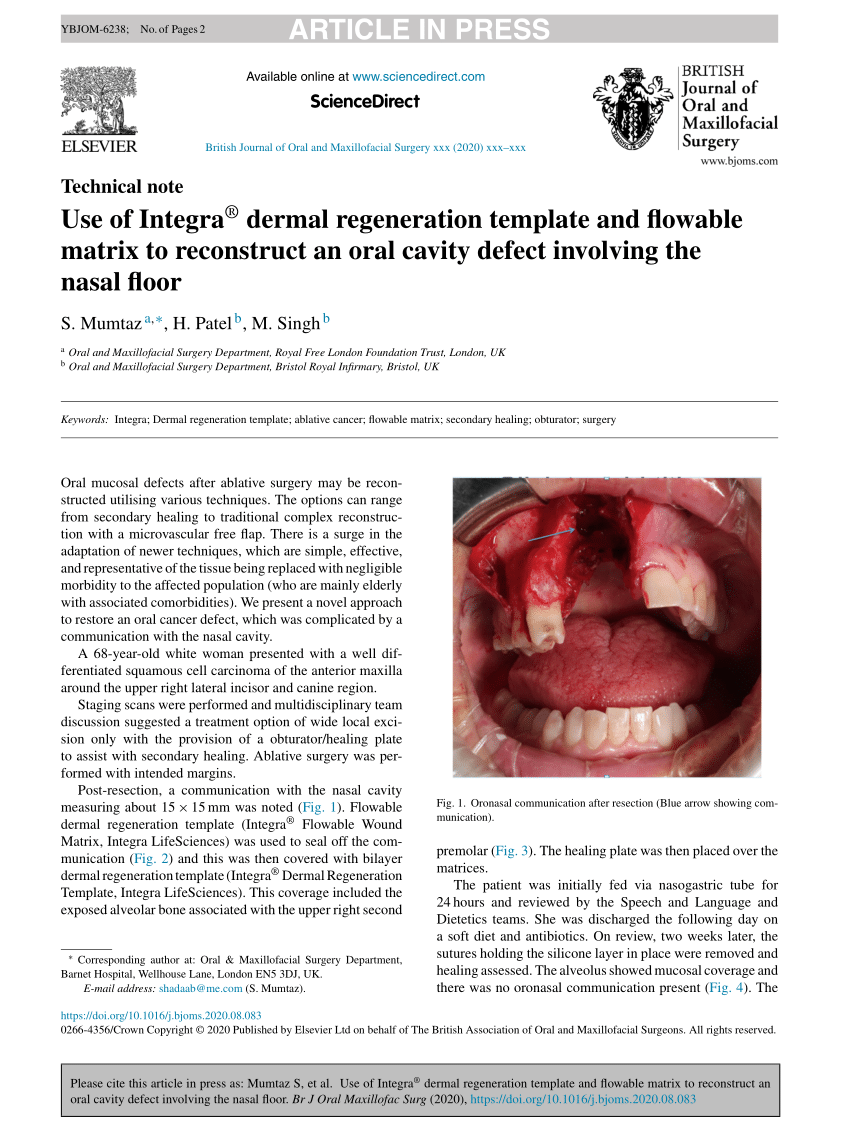
(PDF) Dual INTEGRA® dermal regeneration template and flowable matrix
 for Surgical Wound.png?md=1)
Integra Dermal Regeneration template (IDRT) for Surgical Wound Clinical

Integra Dermal Regeneration Template williamsonga.us
(PDF) The Use of Integra Dermal Regeneration Template Versus Flaps for
Web All Raw Materials Used In The Manufacture Of Integra ® Template, Including Packaging And Accessories, Are Not Formulated With And Do Not Contain Any Natural Rubber Latex Or Dry.
Web Premarket Approval (Pma) Note:
Web Integra ® Template Is Applied To The Excised Wound Bed.
Web Integra® Meshed Dermal Regeneration Template Should Be Applied On The Day Of Excision.
Related Post: