How To Draw The Conjugate Base Of An Acid
How To Draw The Conjugate Base Of An Acid - Acid strength, ka, and p ka. Since there are two places where this proton can be added,. Want to join the conversation? A weak base partially dissociates into hydroxide ions and its conjugate acid at equilibrium. Acid base a.hocl h2o b.hclo4 nh3 c.h2o. Thus nh 3 is called the conjugate base of nh 4+, and nh 4+ is the conjugate acid of nh 3. Web how would you identify the acid, base, conjugate acid, and the conjugate base in the following equation: Autoionization of water and kw. Web how resonance affects the stability of a conjugate base. The strength of the base depends on its rate of. When an acid donates a proton, it forms its. Web how resonance affects the stability of a conjugate base. The strength of the base depends on its rate of. A weak base partially dissociates into hydroxide ions and its conjugate acid at equilibrium. Web a conjugate acid is like its original base partner, but would try to lose its h+. A weak base partially dissociates into hydroxide ions and its conjugate acid at equilibrium. The strength of the base depends on its rate of. Want to join the conversation? Web how would you identify the acid, base, conjugate acid, and the conjugate base in the following equation: Conjugate acid and conjugate base. Acid strength, ka, and p ka. Want to join the conversation? Web how would you identify the acid, base, conjugate acid, and the conjugate base in the following equation: Acid base a.hocl h2o b.hclo4 nh3 c.h2o. A weak base partially dissociates into hydroxide ions and its conjugate acid at equilibrium. Web how resonance affects the stability of a conjugate base. Since there are two places where this proton can be added,. The strength of the base depends on its rate of. When an acid donates a proton, it forms its. A weak base partially dissociates into hydroxide ions and its conjugate acid at equilibrium. When an acid donates a proton, it forms its. Want to join the conversation? Conjugate acid and conjugate base. Acid base a.hocl h2o b.hclo4 nh3 c.h2o. Web how resonance affects the stability of a conjugate base. Thus nh 3 is called the conjugate base of nh 4+, and nh 4+ is the conjugate acid of nh 3. Web how resonance affects the stability of a conjugate base. A weak base partially dissociates into hydroxide ions and its conjugate acid at equilibrium. Want to join the conversation? Since there are two places where this proton can be. Conjugate acid and conjugate base. Autoionization of water and kw. Since there are two places where this proton can be added,. Want to join the conversation? Acid strength, ka, and p ka. Want to join the conversation? Acid base a.hocl h2o b.hclo4 nh3 c.h2o. Since there are two places where this proton can be added,. Web how would you identify the acid, base, conjugate acid, and the conjugate base in the following equation: The strength of the base depends on its rate of. Acid strength, ka, and p ka. Web how would you identify the acid, base, conjugate acid, and the conjugate base in the following equation: Acid base a.hocl h2o b.hclo4 nh3 c.h2o. Web how resonance affects the stability of a conjugate base. Autoionization of water and kw. Web a conjugate acid is like its original base partner, but would try to lose its h+ to return back to basic form. Autoionization of water and kw. When an acid donates a proton, it forms its. Thus nh 3 is called the conjugate base of nh 4+, and nh 4+ is the conjugate acid of nh 3. A weak. A weak base partially dissociates into hydroxide ions and its conjugate acid at equilibrium. When an acid donates a proton, it forms its. Autoionization of water and kw. Want to join the conversation? The strength of the base depends on its rate of. Since there are two places where this proton can be added,. Web a conjugate acid is like its original base partner, but would try to lose its h+ to return back to basic form. Acid strength, ka, and p ka. Want to join the conversation? Acid base a.hocl h2o b.hclo4 nh3 c.h2o. Conjugate acid and conjugate base.
Trick to Find Conjugate Acid and Conjugate Base Ionic Equilibrium

CHEM112 5 6 drawing conjugate acids and bases YouTube
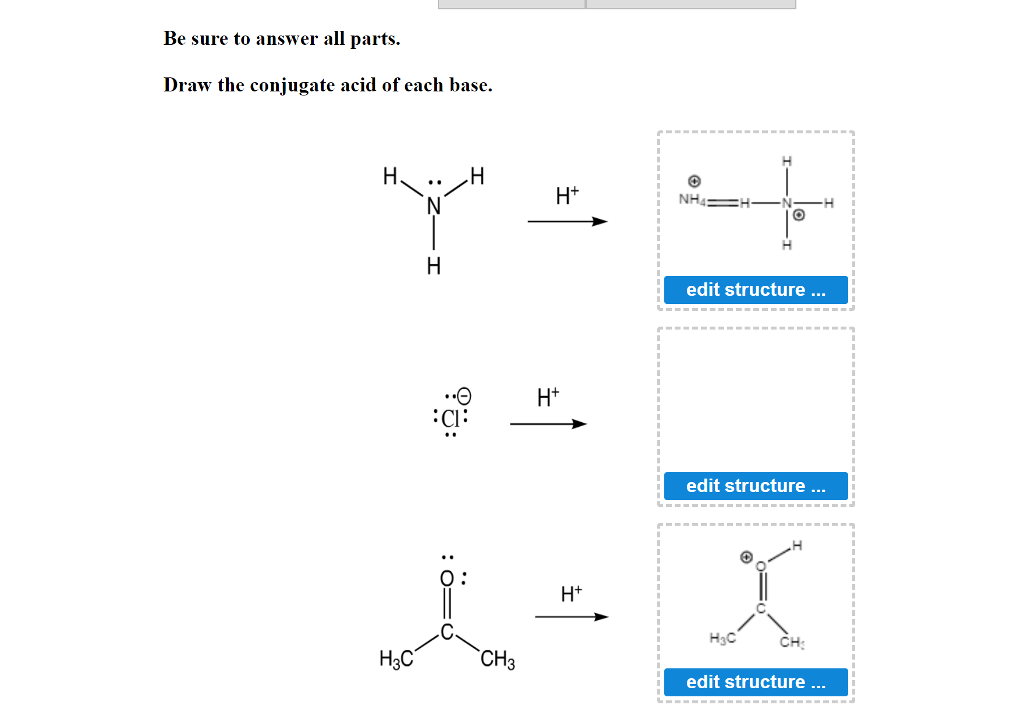
Solved Be sure to answer all parts. Draw the conjugate acid

PPT Acids and Bases PowerPoint Presentation, free download ID1919310
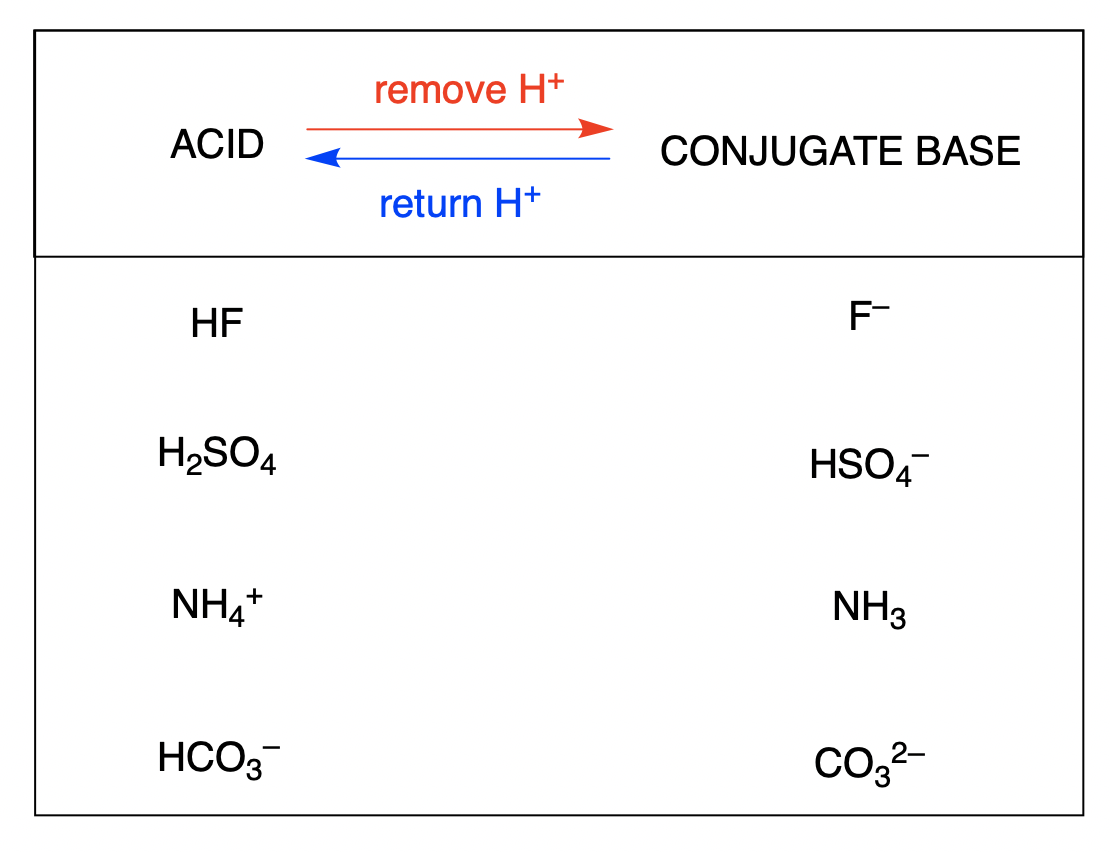
5.1 AcidBase Definitions & Conjugate AcidBase Pairs General

Enter the Conjugate Base for Each Acid.

Acids and Bases (Alevel) ChemistryStudent

Chapter 10 Exercises 3 and 4 Drawing Conjugate Acids and Conjugate

How to Find the Conjugate Acid of a Base Shortcut, Practice Problems
Acids, bases and salts Learning Lab
Web How Resonance Affects The Stability Of A Conjugate Base.
Web How Would You Identify The Acid, Base, Conjugate Acid, And The Conjugate Base In The Following Equation:
Thus Nh 3 Is Called The Conjugate Base Of Nh 4+, And Nh 4+ Is The Conjugate Acid Of Nh 3.
Related Post: