How To Draw Covalent Bonds
How To Draw Covalent Bonds - The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. However, some atoms won’t give up or gain electrons easily. To appreciate how atoms share their valence electrons in covalent bonding. Nonmetals can form a chemical bond by sharing two electrons. The video covers the basic lewis structures you'll see in an introductory chemistry. 0:08 introduction 0:39 h2 1:25 hcl. What are the steps used to draw a lewis structure? Each atom contributes one electron to the bond. Ionic bonding typically occurs when it is easy for one atom to lose one or more electrons and another atom to gain one or more electrons. For example, two hydrogen atoms can form a bond, producing a molecule of h 2. However, some atoms won’t give up or gain electrons easily. Web how do you draw double and triple bonds in lewis symbols? Draw a valid electron dot structure for each of the given elements. How do you write a lewis structure for #ch_3nh_3^+#? Web representing a covalent bond using lewis structures. Understand that covalent compound subscripts are never reduced. The video covers the basic lewis structures you'll see in an introductory chemistry. Ionic bonding typically occurs when it is easy for one atom to lose one or more electrons and another atom to gain one or more electrons. Web how do you draw double and triple bonds in lewis symbols? 1.31. Memorize numerical prefixes used in covalent nomenclature. How do you write a lewis structure for #ch_3nh_3^+#? Illustrate covalent bond formation with lewis electron dot diagrams. Web how to draw covalent bonding molecules. H forms only one bond because it needs only two electrons. Each atom contributes one electron to the bond. 4.7k views 7 years ago 1d: Draw a valid electron dot structure for each of the given elements. Memorize numerical prefixes used in covalent nomenclature. So yes each covalent bond will be a pair of electrons because each atom contributes 1 electron to a bond (and 1+1=2). However, some atoms won’t give up or gain electrons easily. So yes each covalent bond will be a pair of electrons because each atom contributes 1 electron to a bond (and 1+1=2). 0:08 introduction 0:39 h2 1:25 hcl. Web a covalent bond is formed between two atoms by sharing electrons. Web representing a covalent bond using lewis structures. The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. Ionic bonding typically occurs when it is easy for one atom to lose one or more electrons and another atom to gain one or more electrons. However, some atoms won’t give up or gain electrons easily. Illustrate. Hydrogen is an exception to the octet rule. Web a covalent bond is formed between two atoms by sharing electrons. Each atom contributes one electron to the bond. What are the steps used to draw a lewis structure? To appreciate how atoms share their valence electrons in covalent bonding. However, some atoms won’t give up or gain electrons easily. In this video you’ll learn how to draw lewis dot structures for covalent compounds. Illustrate covalent bond formation with lewis electron dot diagrams. How do you write a lewis structure for #ch_3nh_3^+#? The video covers the basic lewis structures you'll see in an introductory chemistry. Hydrogen is an exception to the octet rule. Draw a valid electron dot structure for each of the given elements. Web how to draw covalent bonding molecules. Web 237k views 3 years ago. Illustrate covalent bond formation with lewis electron dot diagrams. The video covers the basic lewis structures you'll see in an introductory chemistry. Web how to draw covalent bonding molecules. 1.31 explain the formation of simple molecular, covalent substances, using dot and cross diagrams, including: Hydrogen is an exception to the octet rule. Illustrate covalent bond formation with lewis electron dot diagrams. The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. Web a covalent bond is formed between two atoms by sharing electrons. Web how do you draw double and triple bonds in lewis symbols? Web to know what types of elements bond to form covalent compounds. Ionic bonding typically occurs when it is easy for one atom to lose one or more electrons and another atom to gain one or more electrons. Web covalent bonds form when two atoms react such that they share electrons in a bond between them and each atom donates half of the electrons which forms the bond from their original valence electrons. Illustrate covalent bond formation with lewis electron dot diagrams. Memorize numerical prefixes used in covalent nomenclature. 1.31 explain the formation of simple molecular, covalent substances, using dot and cross diagrams, including: Understand that covalent compound subscripts are never reduced. What are the steps used to draw a lewis structure? 4.7k views 7 years ago 1d: For example, two hydrogen atoms can form a bond, producing a molecule of h 2. Web how to draw covalent bonding molecules. Using lewis structures, we can represent this as follows: So yes each covalent bond will be a pair of electrons because each atom contributes 1 electron to a bond (and 1+1=2).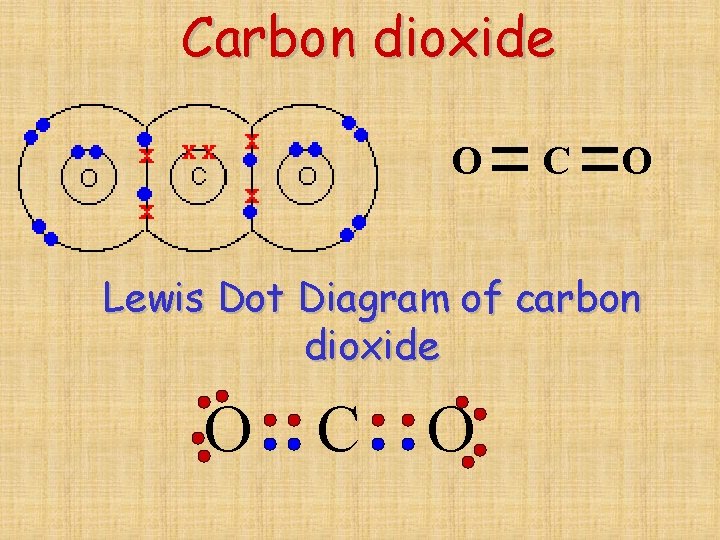
Chapter 8 Covalent Bonding Covalent bonding Usually forms
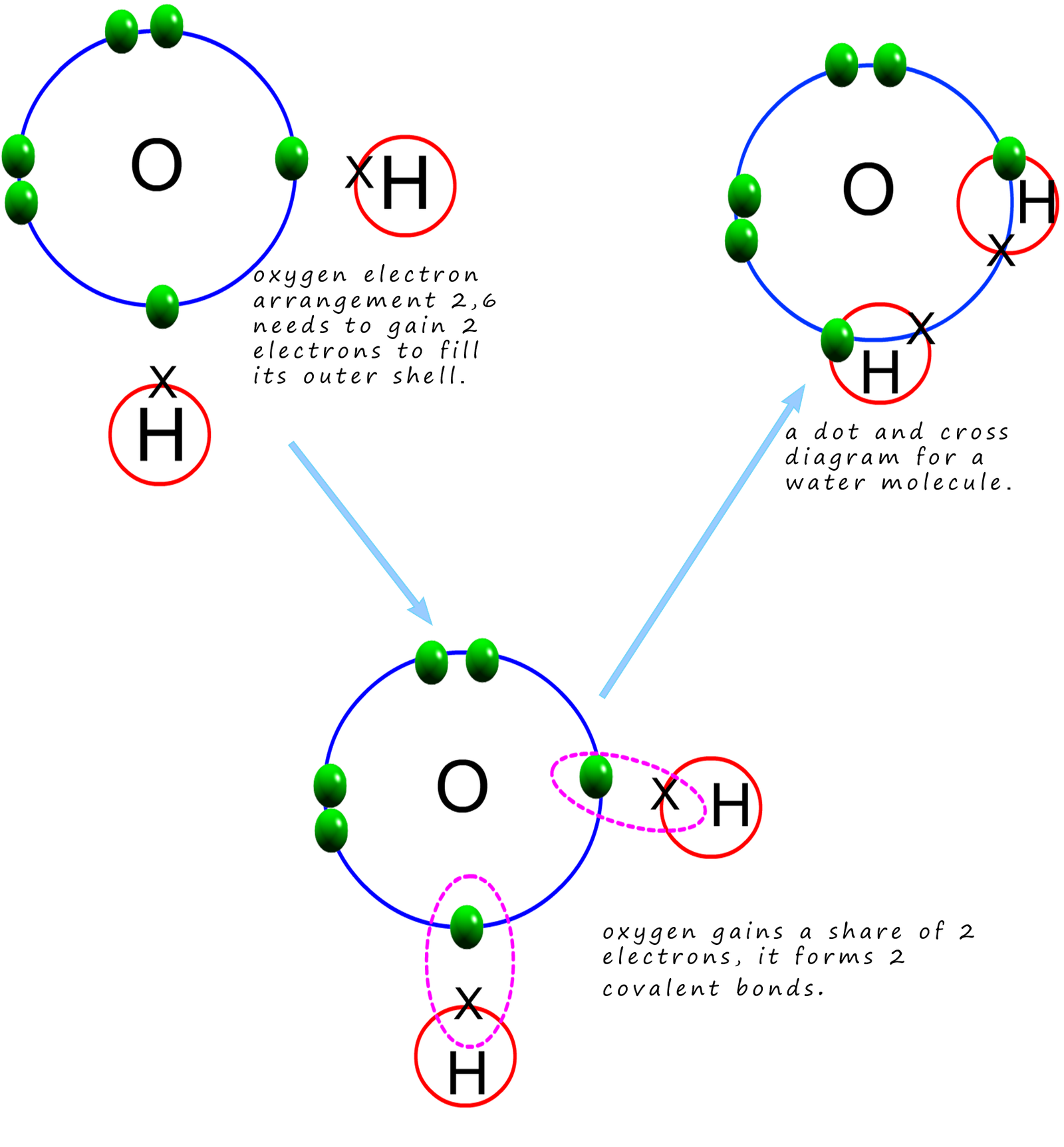
Covalent bonding

Covalent Bonding The Science and Maths Zone

Covalent Bonding The Science and Maths Zone

Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk
Covalent bond (covalency) and its type Overall Science

How is a covalent bond formed

How to Draw Lewis Dot Structure of Covalent Compounds Chemical

How to Draw Covalent Bonds Using Lewis Structures YouTube

CHEMISTRY 101 Draw Lewis dot structures for covalent compounds YouTube
How Do You Write A Lewis Structure For #Ch_3Nh_3^+#?
Hydrogen Is An Exception To The Octet Rule.
H Forms Only One Bond Because It Needs Only Two Electrons.
In This Video You’ll Learn How To Draw Lewis Dot Structures For Covalent Compounds.
Related Post: