How To Draw Atomic Orbitals
How To Draw Atomic Orbitals - Note that the 1s orbitals are significantly lower in energy than the 2s orbitals. Make certain that you can define, and use in context, the key terms below. Web atomic orbitals are the wavefunctions which are solutions of the schroumldinger equation for the hydrogen atom the subset of atomic orbitals and are plotted in three dimensions to exhibit their characteristic shapes the orbitals are drawn by showing their boundary surfaces in the second view and signs are attached to the. With oxygen, you know that the atomic orbital potential energies go in the following order: An orbital is a space where a specific pair of electrons can be found. Web the goal of this section is to understand the electron orbitals (location of electrons in atoms), their different energies, and other properties. Web for a given atom, the s orbitals also become higher in energy as n increases because of their increased distance from the nucleus. For the p orbitals, draw one arrow pointing up on each of the lines first. Web orbital diagrams use the same basic format, but instead of numbers for the electrons, they use ↑ and ↓ arrows, as well as giving each orbital its own line, to represent the spins of the electrons too. Let's learn about the shapes of atomic orbitals in this. Web the goal of this section is to understand the electron orbitals (location of electrons in atoms), their different energies, and other properties. Web atomic orbitals are the wavefunctions which are solutions of the schroumldinger equation for the hydrogen atom the subset of atomic orbitals and are plotted in three dimensions to exhibit their characteristic shapes the orbitals are drawn. The use of quantum theory provides the best understanding to these topics. Web fill each orbital (horizontal line) with the number of occupant electrons. Web © 2024 google llc. Orbital diagrams are a visual way to show where the electrons are located within an atom. Web we predict the distribution of electrons in these molecular orbitals by filling the orbitals. Web © 2024 google llc. Electron configurations are expressed through a notation that looks like this: Get a 10 bullets summary of the topic. Web 230313 how to draw shapes of orbitals. You need to know what type of pi electron contribution each type of. To understand the 3d representation of electronic orbitals. The 7 rules of drawing molecular orbitals. Web atomic orbitals are the wavefunctions which are solutions of the schroumldinger equation for the hydrogen atom the subset of atomic orbitals and are plotted in three dimensions to exhibit their characteristic shapes the orbitals are drawn by showing their boundary surfaces in the second. Describe the physical significance of an orbital. Web fill each orbital (horizontal line) with the number of occupant electrons. Web orbital diagrams use the same basic format, but instead of numbers for the electrons, they use ↑ and ↓ arrows, as well as giving each orbital its own line, to represent the spins of the electrons too. 1s 2 2s. Web © 2024 google llc. Web fill each orbital (horizontal line) with the number of occupant electrons. Web we predict the distribution of electrons in these molecular orbitals by filling the orbitals in the same way that we fill atomic orbitals, by the aufbau principle. List the atomic orbitals from 1 s to 3 d in order of increasing energy.. Web 230313 how to draw shapes of orbitals. Web © 2024 google llc. This organic chemistry video tutorial explains the hybridization of atomic orbitals. You need to know what type of pi electron contribution each type of. In this case, sulfur has 16 electrons that need to be placed into orbitals. Web © 2024 google llc. We're going to look at what orbitals are, what they represent, how electrons go in orbitals, the order electrons go in orbitals, and the shapes of. So the atomic orbital diagram is simply those orbitals in that order of energy. Get a 10 bullets summary of the topic. Here are the 7 rules you need. I will use oxygen ( o2(g)) as an example. Web 1) look at the periodic table to see how many electrons sulfur has. Make certain that you can define, and use in context, the key terms below. I will use oxygen (o_2 (g)) as an example. 2) looking at our cheat sheet, draw the orbitals one at a time, adding. An orbital is a space where a specific pair of electrons can be found. After completing this section, you should be able to. Atomic orbitals and their energies. It discusses how to determine the. This is also due to the history when they were discovered. With oxygen, you know that the atomic orbital potential energies go in the following order: Describe the physical significance of an orbital. This video goes over how to properly draw orbital diagrams for an element, after determining the electron configuration. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. List the atomic orbitals from 1 s to 3 d in order of increasing energy. I will use oxygen (o_2 (g)) as an example. Web atomic orbitals are the wavefunctions which are solutions of the schroumldinger equation for the hydrogen atom the subset of atomic orbitals and are plotted in three dimensions to exhibit their characteristic shapes the orbitals are drawn by showing their boundary surfaces in the second view and signs are attached to the. You need to know what type of pi electron contribution each type of. Web 1) look at the periodic table to see how many electrons sulfur has. The aufbau principle, the pau. To understand the 3d representation of electronic orbitals. Web for a given atom, the s orbitals also become higher in energy as n increases because of their increased distance from the nucleus. So the atomic orbital diagram is simply those orbitals in that order of energy. This knowledge is a precursor to chemical bonding. Orbital diagrams must follow 3 rules: For the s orbitals, first draw an arrow pointing up and an arrow pointing down.
Radial and Angular Parts of Atomic Orbitals Chemistry LibreTexts

Solved ?1. Consider the s, p, and d atomic orbitals as
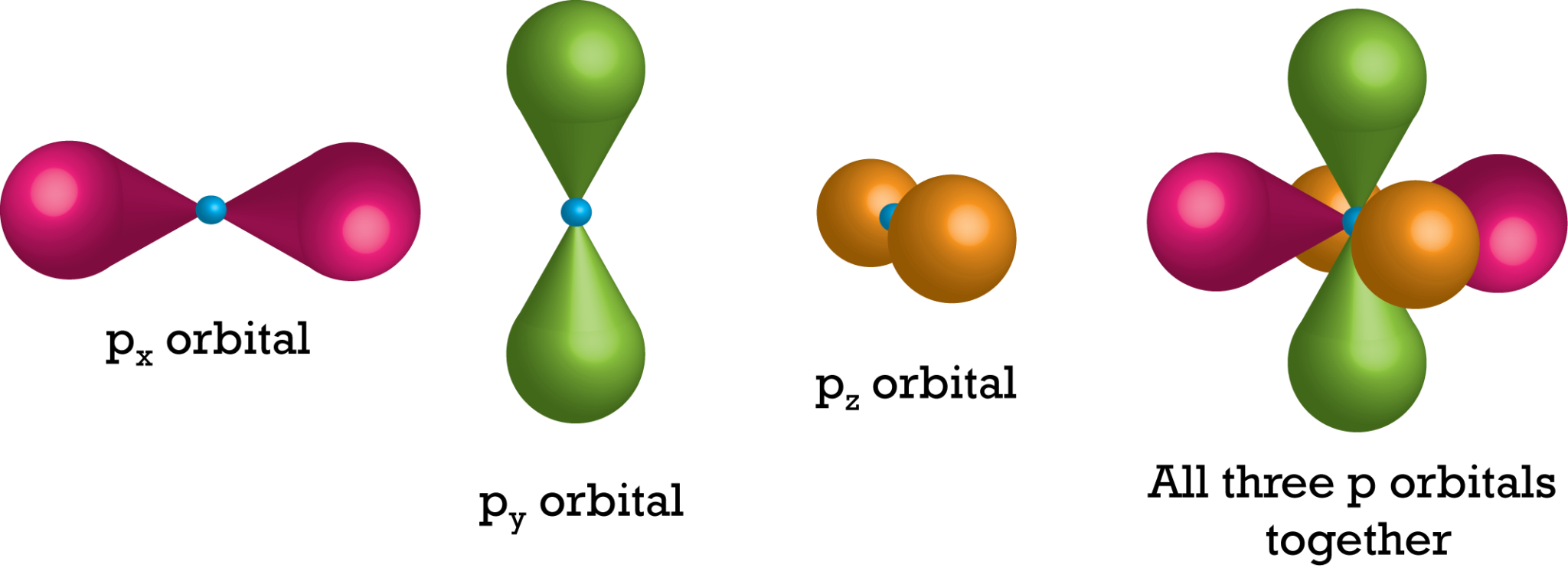
Shapes of Orbitals and their Types Chemistry Skills
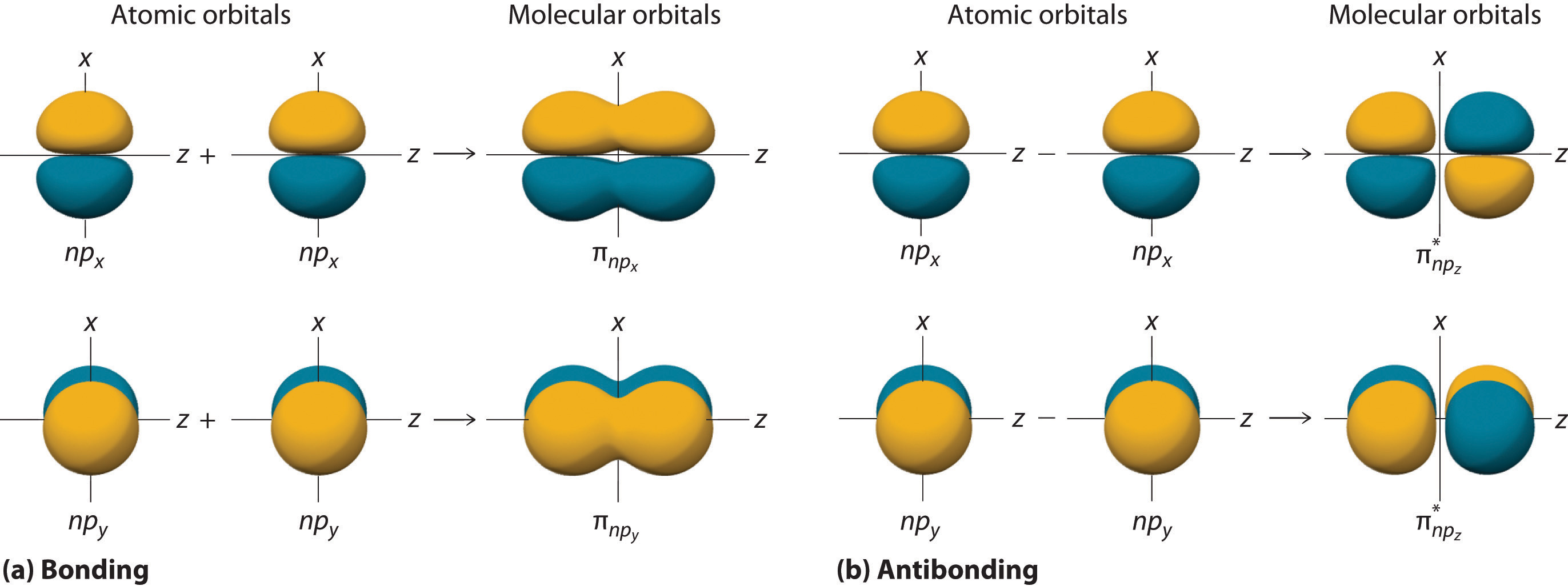
3.1.7 Molecular Orbitals Chemistry LibreTexts

Shapes of Atomic Orbitals — Overview & Examples Expii

Biochemistry Glossary Orbitals 2. Shape Draw It to Know It

3.7 Electron Arrangement The Quantum Model Chemistry LibreTexts

6.6 The Shapes of Atomic Orbitals Chemistry LibreTexts

Chemical bonding Atomic Orbitals, Shapes, Hybridization Britannica

Shapes of Atomic Orbitals — Overview & Examples Expii
This Organic Chemistry Video Tutorial Explains The Hybridization Of Atomic Orbitals.
Web © 2024 Google Llc.
An Orbital Is A Space Where A Specific Pair Of Electrons Can Be Found.
V_ (1S) << V_ (2S) < V_ (2P) So The Atomic Orbital Diagram Is Simply Those Orbitals In That Order Of Energy.
Related Post: