Drawing Molecular Orbital
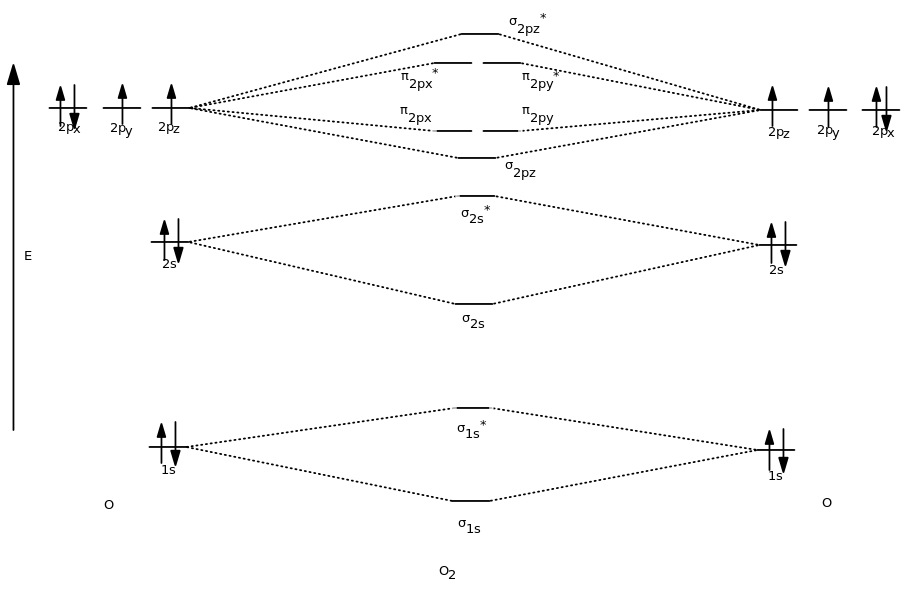
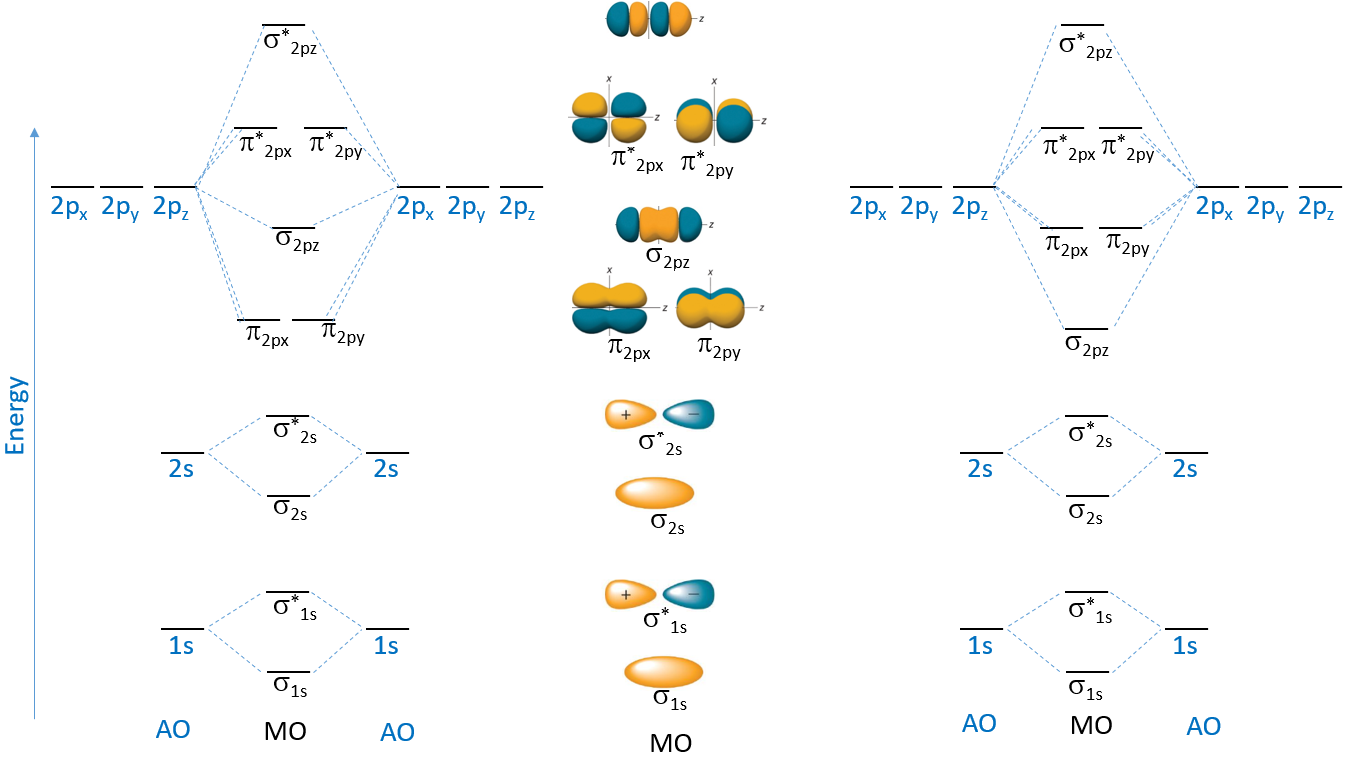
Drawing Molecular Orbital - Web describe the essential difference between a sigma and a pi molecular orbital. Molecular orbital theory is a more sophisticated model for understanding the nature of chemical bonding. V 1s << v 2s < v 2p. How to draw pi molecular orbitals for a given pi system. There are some departures from that rule, sometimes, but that's the simplest place to start. Both h a 2 a + and h a 2 a − do not exist. Which among the following is true with respect to the given species? Both h a 2 a + and h a 2 a − are equally stable. Web drawing out the molecular orbitals of each of these systems can be done in a stepwise manner. Lines, which are often dashed diagonal lines, connect mo levels to their constituent ao levels. Molecular orbital theory is a more sophisticated model for understanding the nature of chemical bonding. Web how might one draw atomic and molecular orbital diagrams? Both h a 2 a + and h a 2 a − are equally stable. Web to a great extent, the order of molecular orbitals in energy can be considered to follow from the order. Web molecular orbital theory; We learned some key lessons for drawing out the molecular orbitals of (linear) pi systems that i will quickly rehash here. Solution we draw a molecular orbital energy diagram similar to that shown in figure 8.37. This gives you the total number of electrons you will have to distribute among the molecular orbitals you form. Web. Web how to draw molecular orbital diagrams for conjugated systems. Lines, which are often dashed diagonal lines, connect mo levels to their constituent ao levels. How to draw a molecular orbital diagram. Web drawing out the molecular orbitals of each of these systems can be done in a stepwise manner. Both h a 2 a + and h a 2. Web molecular orbital diagrams. There are some departures from that rule, sometimes, but that's the simplest place to start. Web the objective of this wiki is to provide readers with the fundamental steps in constructing simple homonuclear and heteronuclear diatomic molecular orbital diagrams. Determine the atomic orbitals of your atoms. How to draw pi molecular orbitals for a given pi. Determine how many valence electrons you have on each atom (you can ignore the core electrons as core orbitals contribute little to molecular orbitals). Web drawing molecular orbital diagrams. Web the objective of this wiki is to provide readers with the fundamental steps in constructing simple homonuclear and heteronuclear diatomic molecular orbital diagrams. Previously we’ve looked at the molecular orbitals. There are some departures from that rule, sometimes, but that's the simplest place to start. Both h a 2 a + and h a 2 a − do not exist. Solution we draw a molecular orbital energy diagram similar to that shown in figure 8.37. Web how to draw molecular orbital diagrams for conjugated systems. Web molecular orbital diagrams. So the atomic orbital diagram is simply those orbitals in that order of energy. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. From this diagram, calculate the bond order. Molecular orbital diagrams show molecular orbital (mo) energy levels in the centre, surrounded by constituent atomic orbital (ao) energy levels for comparison, and the energy levels increase from bottom to top. Web describe the essential difference between a sigma and a pi molecular orbital. It describes the formation of bonding and antibonding molecular orbitals from the combination of. Valence bond. Molecular orbital theory is a more sophisticated model for understanding the nature of chemical bonding. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. Both h a 2 a + and h a 2 a − are equally stable. How to draw a molecular orbital diagram. Valence bond theory is able to explain many aspects of bonding,. How does this diagram account for the paramagnetism of o 2? I will use oxygen ( o2(g)) as an example. H a 2 a + and h a 2 a −. Both h a 2 a + and h a 2 a − are equally stable. The 2s orbitals on one atom combine with the 2s orbitals on another to. It describes the formation of bonding and antibonding molecular orbitals from the combination of. Web molecular orbital diagrams are complex, involving two additional orbitals, electronegativity, atomic symmetries and atomic energies. How does this diagram account for the paramagnetism of o 2? Molecular orbital diagrams show molecular orbital (mo) energy levels in the centre, surrounded by constituent atomic orbital (ao) energy levels for comparison, and the energy levels increase from bottom to top. Web drawing out the molecular orbitals of each of these systems can be done in a stepwise manner. V 1s << v 2s < v 2p. Solution we draw a molecular orbital energy diagram similar to that shown in figure 8.37. Web molecular orbital diagrams, bond order, and number of unpaired electrons draw the molecular orbital diagram for the oxygen molecule, o 2. Previously we’ve looked at the molecular orbitals of the allyl system, and of butadiene. Determine how many valence electrons you have on each atom (you can ignore the core electrons as core orbitals contribute little to molecular orbitals). This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion h 2 +. H a 2 a + and h a 2 a −. Valence bond theory is able to explain many aspects of bonding, but not all. Web molecular orbital theory; Web drawing molecular orbital diagrams. Define bond order, and state its significance.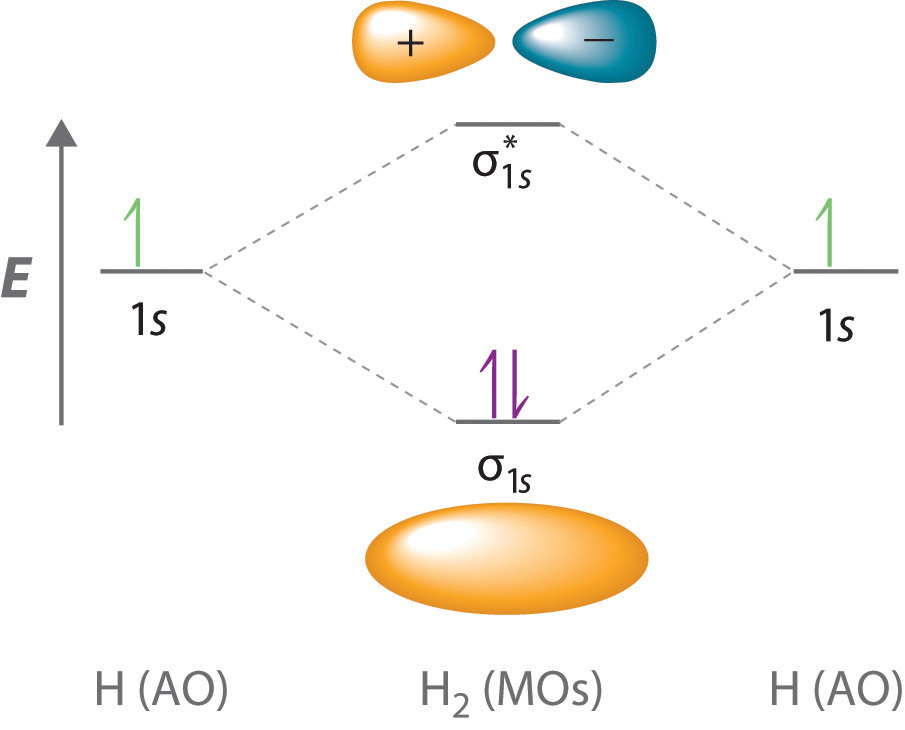
4.9 Molecular Orbitals Chemistry LibreTexts

Molecular Orbital Theory Chemistry
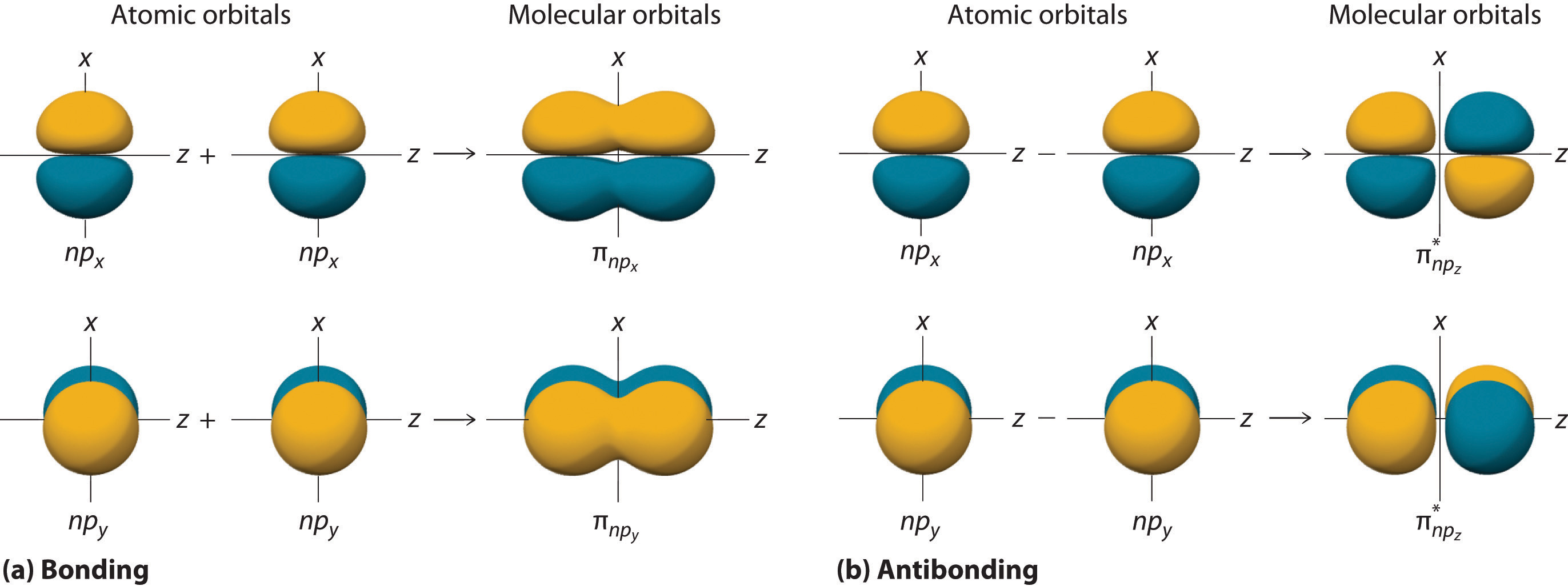
Drawing Atomic and Molecular Orbitals Diagrams for Molecules Organic
9.7 Molecular Orbitals Chemistry LibreTexts

The Pi Molecular Orbitals of Butadiene And How To Draw Them
9.3 Molecular Orbital Theory Chemistry LibreTexts
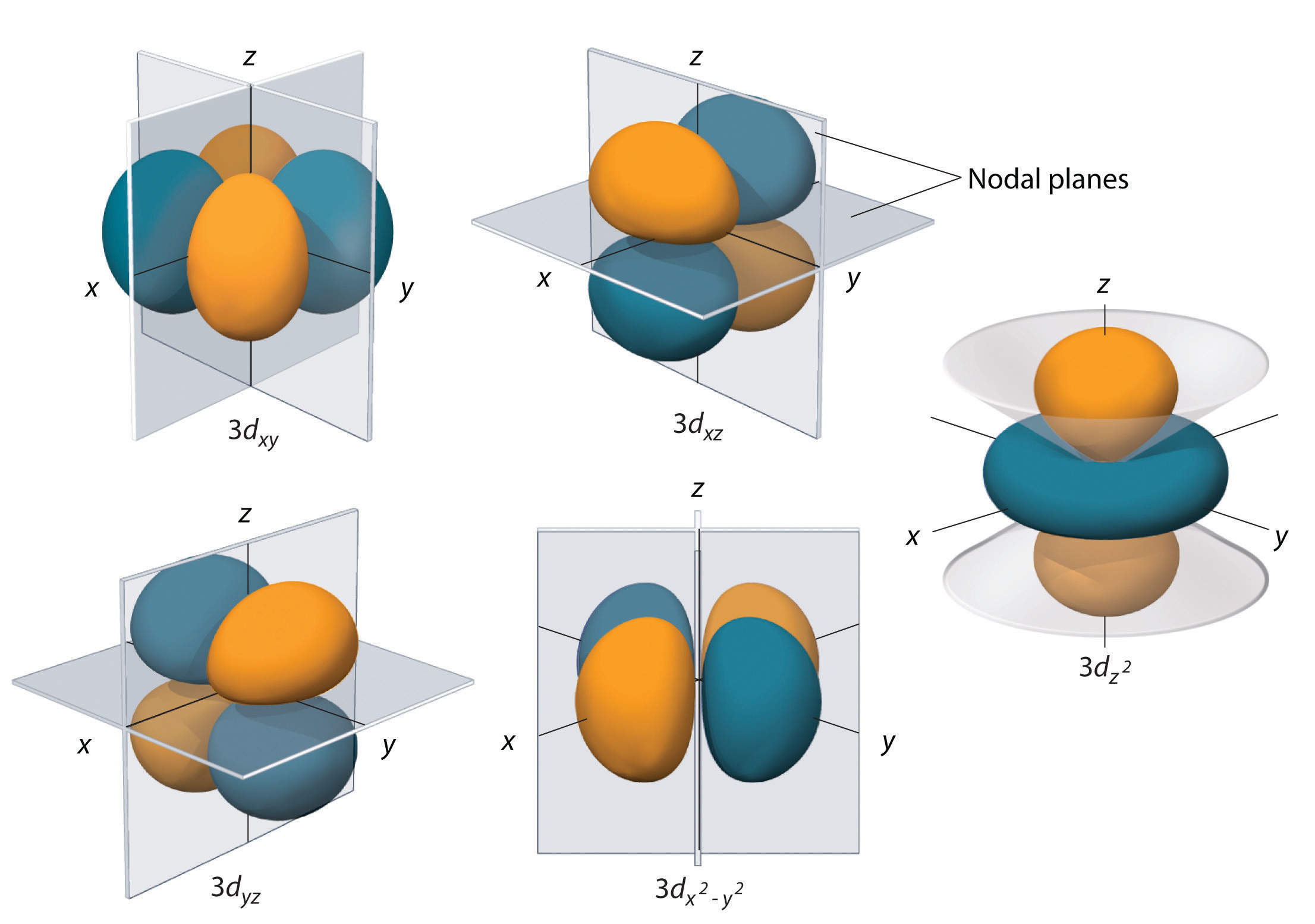
6.6 3D Representation of Orbitals Chemistry LibreTexts

Molecular Orbital Diagrams simplified by Megan Lim Medium

8.3 Development of Quantum Theory CHEM 1114 Introduction to Chemistry

Molecular Orbital Diagrams 101 Diagrams
The 2S Orbitals On One Atom Combine With The 2S Orbitals On Another To Form A 2S Bonding And A 2S * Antibonding Molecular Orbital, Just Like The 1S And 1S * Orbitals Formed From The 1S Atomic Orbitals.
Web This Chemistry Video Tutorial Provides A Basic Introduction Into Molecular Orbital Theory.
Web Describe The Essential Difference Between A Sigma And A Pi Molecular Orbital.
If We Arbitrarily Define The Z Axis Of The Coordinate System For The O 2 Molecule As The Axis Along Which The Bond.
Related Post: