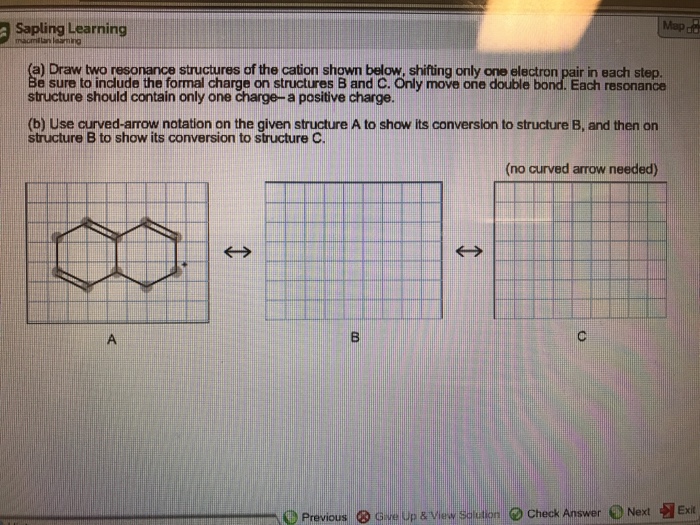
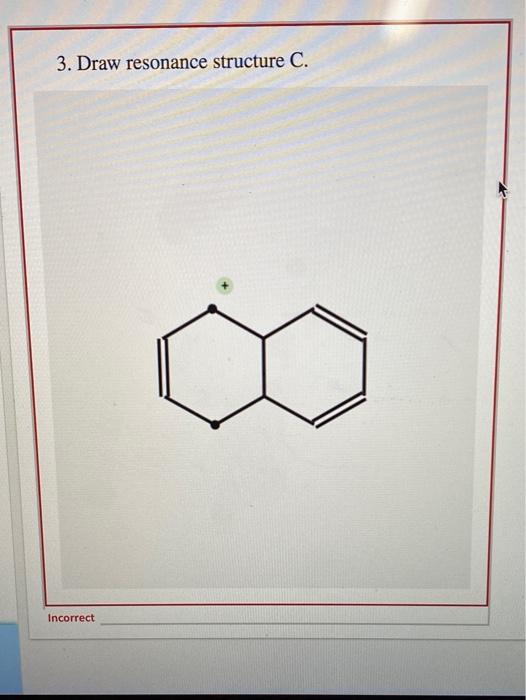
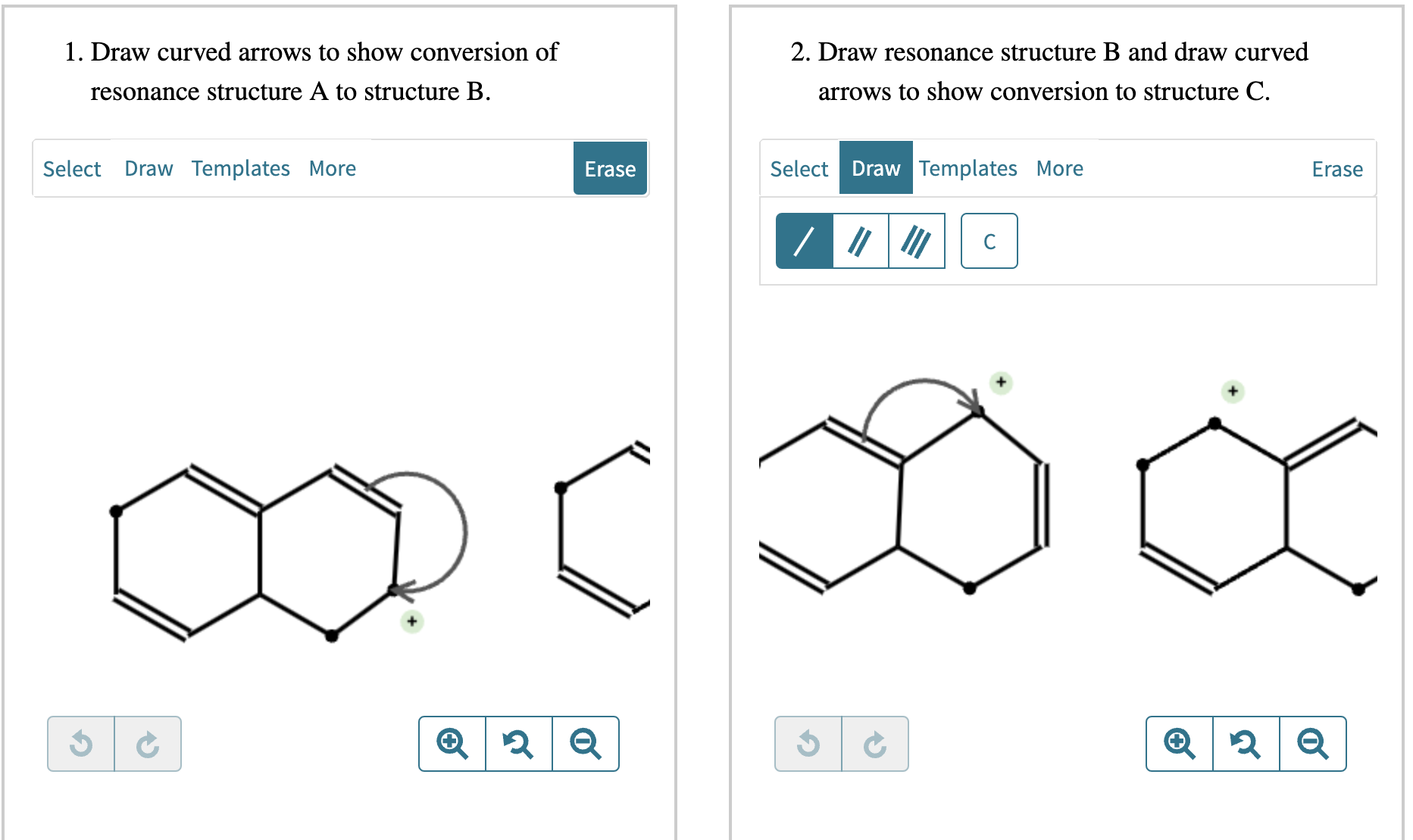
Draw Two Resonance Structures Of The Cation Shown
Draw Two Resonance Structures Of The Cation Shown - Web resonance contributors do not have to be equivalent. Draw both resonance structures of the most stable carbocation intermediate in the reaction shown. Web draw all possible resonance structures for the following free radical: Use formal charges to determine. Web in this tutorial on resonance structures, you will learn what resonance structures are and how to find all of the possible resonance structures a molecule has. Be sure to include the formal charge on structures b. Web in chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures into. Be sure to include the formal charge on structures b and c.(b) use. Draw two resonance structures of the cation shown below, shifting only one electron pair in each step. Determine the relative stability of resonance structures using a set of rules. Web draw all possible resonance structures for the following free radical: Web to draw all resonance structures, take the lewis structure we drawn by using vespr rule. Web resonance contributors do not have to be equivalent. Web draw the resonance structure indicated by the curved arrows. + hbr • you do not have to consider. Determine all the pushable electron pairs and the places where the electrons. Sometimes one dot structures is not enough to completely describe a molecule or an ion, sometimes you need two or more, and here's an example: Web in this tutorial on resonance structures, you will learn what resonance structures are and how to find all of the possible resonance. Three resonance structures are possible for the cation shown. Web to draw all resonance structures, take the lewis structure we drawn by using vespr rule. In resonance structures, it does not require to show transformation of electrons by. Draw two resonance structures of the cation shown below, shifting only one electron pair in each step. Because of this, resonance structures. One resonance form is given. Determine all the pushable electron pairs and the places where the electrons. Web explain the concept of resonance and draw lewis structures representing resonance forms for a given molecule Web draw the resonance structures of molecules or ions that exhibit delocalization. Determine the relative stability of resonance structures using a set of. Three resonance structures are possible for the cation shown. Draw two resonance structures of the cation shown below, shifting only one electron pair in each step. Sometimes one dot structures is not enough to completely describe a molecule or an ion, sometimes you need two or more, and here's an example: Web to draw all resonance structures, take the lewis. Sometimes one dot structures is not enough to completely describe a molecule or an ion, sometimes you need two or more, and here's an example: Determine the relative stability of resonance structures using a set of. Web draw the resonance structures of molecules or ions that exhibit delocalization. Web draw the resonance structure indicated by the curved arrows. Web draw. Web draw the resonance structure indicated by the curved arrows. Determine the relative stability of resonance structures using a set of rules. Because of this, resonance structures do necessarily contribute equally to the resonance hybrid. Be sure to include the formal charge on structures b and c.(b) use. Determine all the pushable electron pairs and the places where the electrons. Use formal charges to determine. Determine all the pushable electron pairs and the places where the electrons. Web in this tutorial on resonance structures, you will learn what resonance structures are and how to find all of the possible resonance structures a molecule has. In resonance structures, it does not require to show transformation of electrons by. + hbr •. Use resonance structures to show electron delocalization. Assign formal charges, and evaluate which of the two structures is a larger contributor to the resonance hybrid. Use formal charges to determine. Because of this, resonance structures do necessarily contribute equally to the resonance hybrid. Be sure to include the formal charge on structures b and c.(b) use. Use resonance structures to show electron delocalization. Web resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single lewis. Because of this, resonance structures do necessarily contribute equally to the resonance hybrid. Draw both resonance structures of the most stable carbocation intermediate in the reaction shown. Web. Be sure to include the formal charge on structures b and c. + hbr • you do not have to consider. Web draw the resonance structure indicated by the curved arrows. Web in chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures into. In resonance structures, it does not require to show transformation of electrons by. Determine the relative stability of resonance structures using a set of rules. Use resonance structures to show electron delocalization. One resonance form is given. Web (a) draw two resonance structures of the cation shown below, shifting only one electron pair in each step. Web to draw all resonance structures, take the lewis structure we drawn by using vespr rule. Web resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single lewis. Web (a) draw two resonance structures of the cation shown below, shifting only one electron pair in each step. Web explain the concept of resonance and draw lewis structures representing resonance forms for a given molecule Be sure to include the formal charge on structures b and c.(b) use. Draw both resonance structures of the most stable carbocation intermediate in the reaction shown. Use formal charges to determine.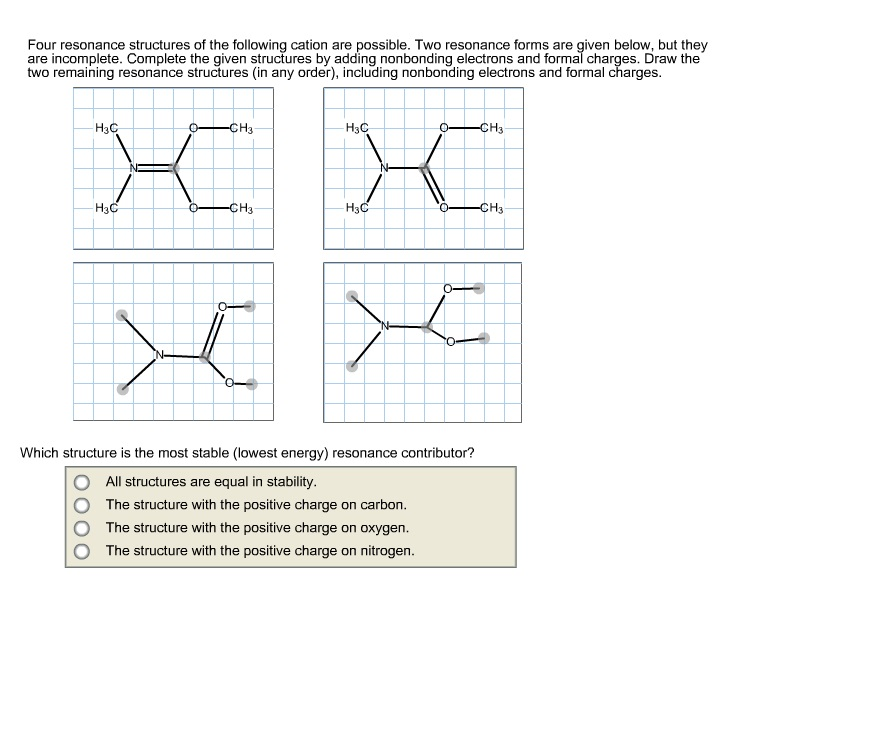
draw two resonance structures of the cation shown below

(a) Draw two resonance structures of the cation shown below, shifting
Solved Draw Two Resonance Structures Of The Cation Shown
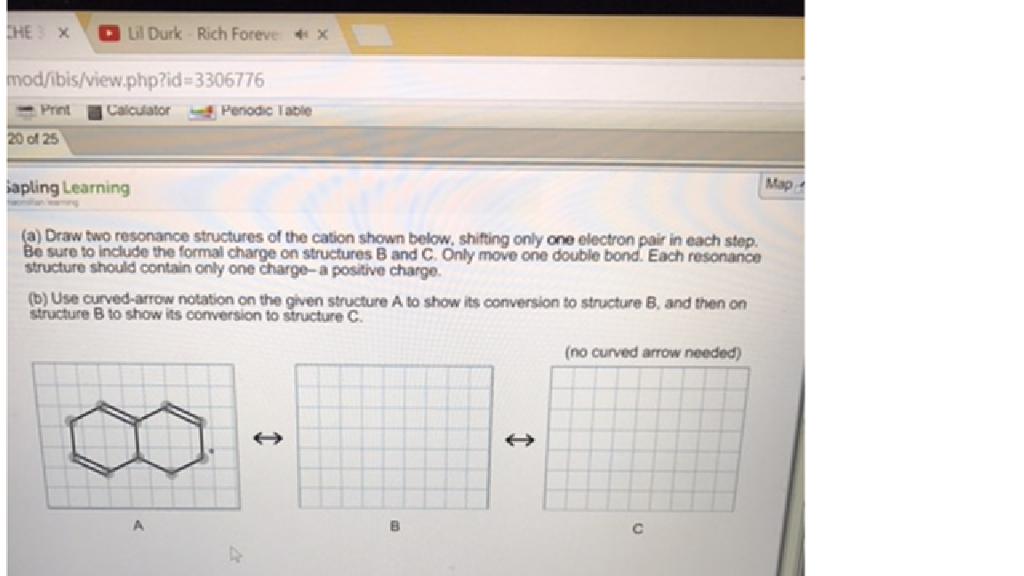
Solved Draw two resonance structures of the cation shown
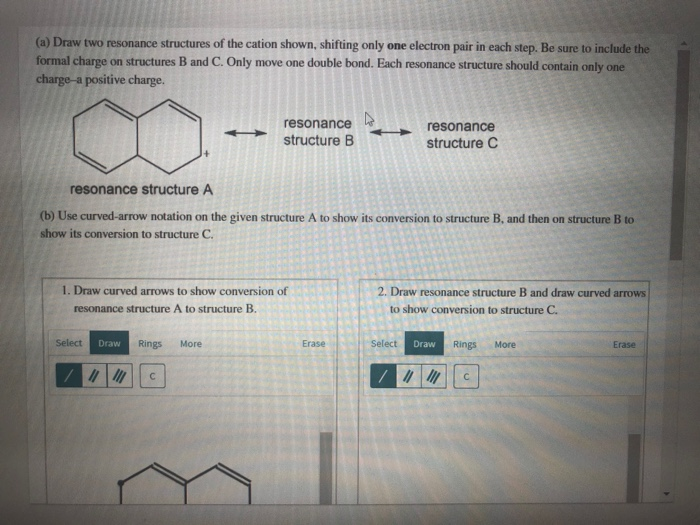
draw two resonance structures of the cation shown below blackbodyart
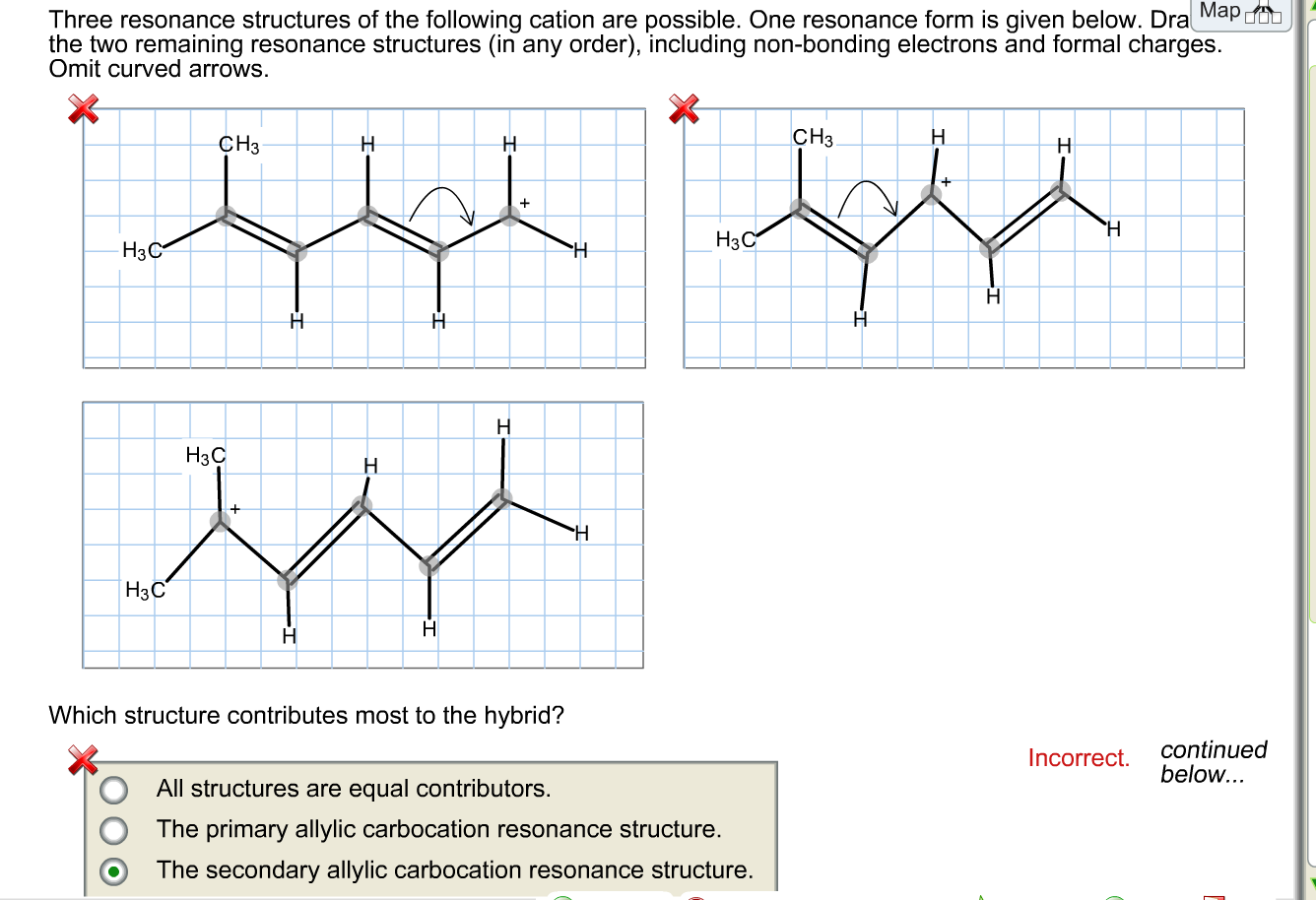
draw two resonance structures of the cation shown below blackbodyart
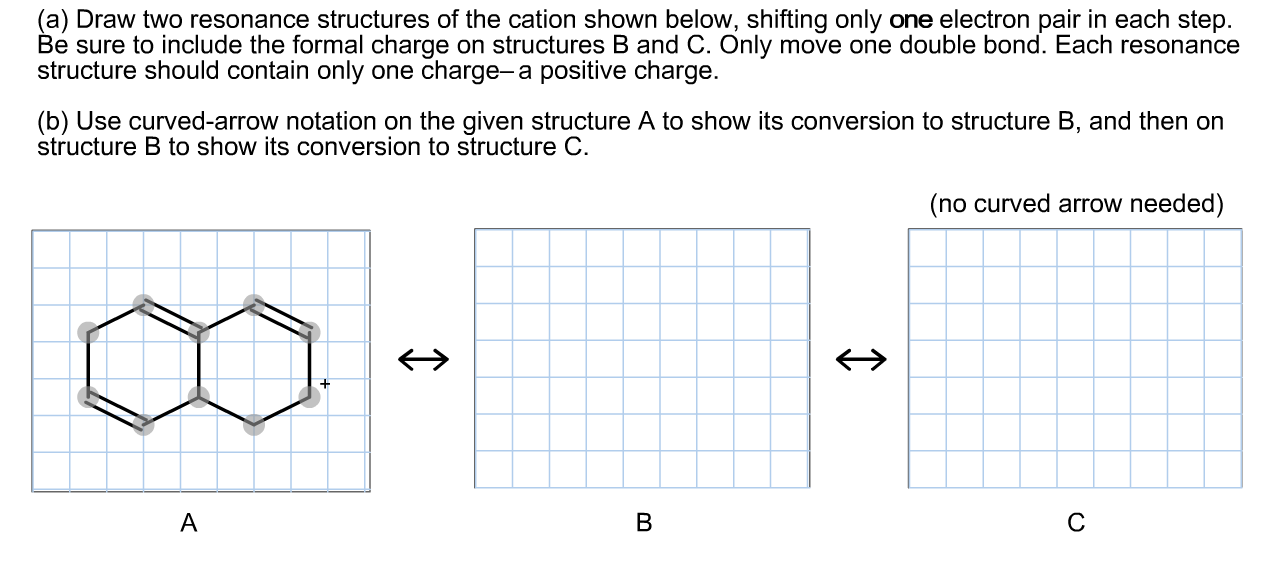
Solved (a) Draw two resonance structures of the cation shown
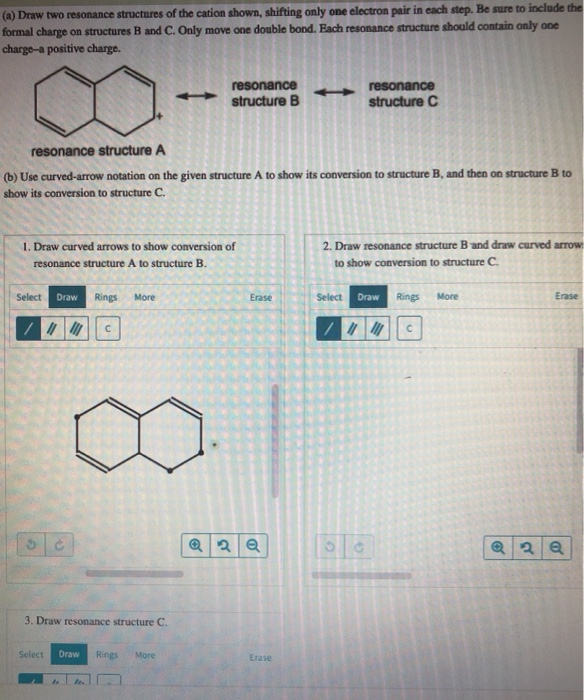
Solved (a) Draw two resonance structures of the cation
Solved (a) Draw two resonance structures of the cation
Solved Draw two resonance structures of the cation shown,
Sometimes One Dot Structures Is Not Enough To Completely Describe A Molecule Or An Ion, Sometimes You Need Two Or More, And Here's An Example:
Web Draw All Possible Resonance Structures For The Following Free Radical:
Determine The Relative Stability Of Resonance Structures Using A Set Of.
Determine All The Pushable Electron Pairs And The Places Where The Electrons.
Related Post: