Draw The Structure Of Ch2O Including Lone Pairs
Draw The Structure Of Ch2O Including Lone Pairs - Click on a bond to cycle through single, double, and triple. In order to draw the lewis structure of ch2o, first of all you have to find the total number of valence electrons present in the ch2o molecule. Total electron pairs are determined by dividing the number total valence electrons by two. Determine the total number of electron pairs that exist as lone pairs and bonds. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Carbon (c) is the least electronegative atom in the ch2o lewis structure and. Determine the total number of valence electrons: Count the total number of valence electrons: Web draw the structure of ch2o including lone pairs. Let's do the lewis structure for ch2o, methanal or formaldehyde. Start by focusing on the structure of the first molecule, ch2o; Web draw the structure of ch2o including lone pairs. Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells. Web total valence electrons = 2 + 6 + 4 = 12. Web here’s how you can easily draw the ch 2 o lewis. Web the structure on the right is the lewis electron structure, or lewis structure, for h2o. Total valence electrons available for drawing the lewis structure of ch 2 o = 4 + 1 (2) + 6 = 12 valence electrons. The steric number for the carbon central atom is 3, hence, the sp2 hybridization types formed in the ch2o molecule.. Total electron pairs are determined by dividing the number total valence electrons by two. Count the total number of valence electrons: So, the total number of valence electrons in ch2o is: Web there are a total of 4 lone pairs of electrons and 8 shared pairs of electrons present in the lewis structure of ch2o. This is a popular solution! Let's do the lewis structure for ch2o, methanal or formaldehyde. Click once on the grid, then just start drawing (dick, hold, and drag). Lewis structure is a pictorial representation of the atoms in the molecules, their bonds, and lone pairs of electrons. Total electron pairs are determined by dividing the number total valence electrons by two. Web here’s how you. In order to draw the lewis structure of ch2o, first of all you have to find the total number of valence electrons present in the ch2o molecule. Web draw the structure of ch2o including lone pairs. Here, the given molecule is ch2o. = 12 valence electrons of ch2o. You'll get a detailed solution from a subject matter expert that helps. Draw the lewis structure for formaldehyde, ch,o. Calculate the total number of valence electrons. In order to draw the lewis structure of ch2o, first of all you have to find the total number of valence electrons present in the ch2o molecule. Web for a molecular formula of ch2o, we would have 12 valence electrons to distribute around the molecule, or. See the big list of lewis structures. For, hcho molecule, total pairs of electrons are six in their valence shells. Draw the structure of ch 2 o, including lone pairs. Draw the lewis structure for formaldehyde, ch,o. Co2 сн,он ch2o hcooh ch4 b) in which of these compounds is the extent of oxidation the. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Draw the lewis structures of the following molecules. View the full answer step 2. Click on a bond to cycle through single, double, and triple. Looking at the periodic table, carbon has 4. A) for each of the following compounds, draw the lewis structure. Draw the structure of ch 2 o, including lone pairs. #3 if needed, mention formal charges on the atoms. 4 (c) + 2 (h) + 6 (o) = 12. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. So, the total number of valence electrons in ch2o is: #2 mention lone pairs on the atoms. 6 steps to draw the lewis structure of ch2o. See the big list of lewis structures. Web the total number of valence electrons in oxygen is six. The total number of valence electrons in hydrogen is one. 100% formaldehyde, ch 2 o, is used as an embalming agent. Web © 2024 google llc. A) for each of the following compounds, draw the lewis structure. For, hcho molecule, total pairs of electrons are six in their valence shells. See the big list of lewis structures. Co2 сн,он ch2o hcooh ch4 b) in which of these compounds is the extent of oxidation the. So, the total number of valence electrons in ch2o is: Thus, ch2o has a total of twelve valence electrons that can help in drawing its lewis structure. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. The steric number for the carbon central atom is 3, hence, the sp2 hybridization types formed in the ch2o molecule. Web web here’s how you can easily draw the ch 2 o lewis structure step by step: In order to draw the lewis structure of ch2o, first of all you have to find the total number of valence electrons present in the ch2o molecule. Determine the total number of valence electrons: Web the total number of valence electrons in oxygen is six. Include lone pairs of electrons.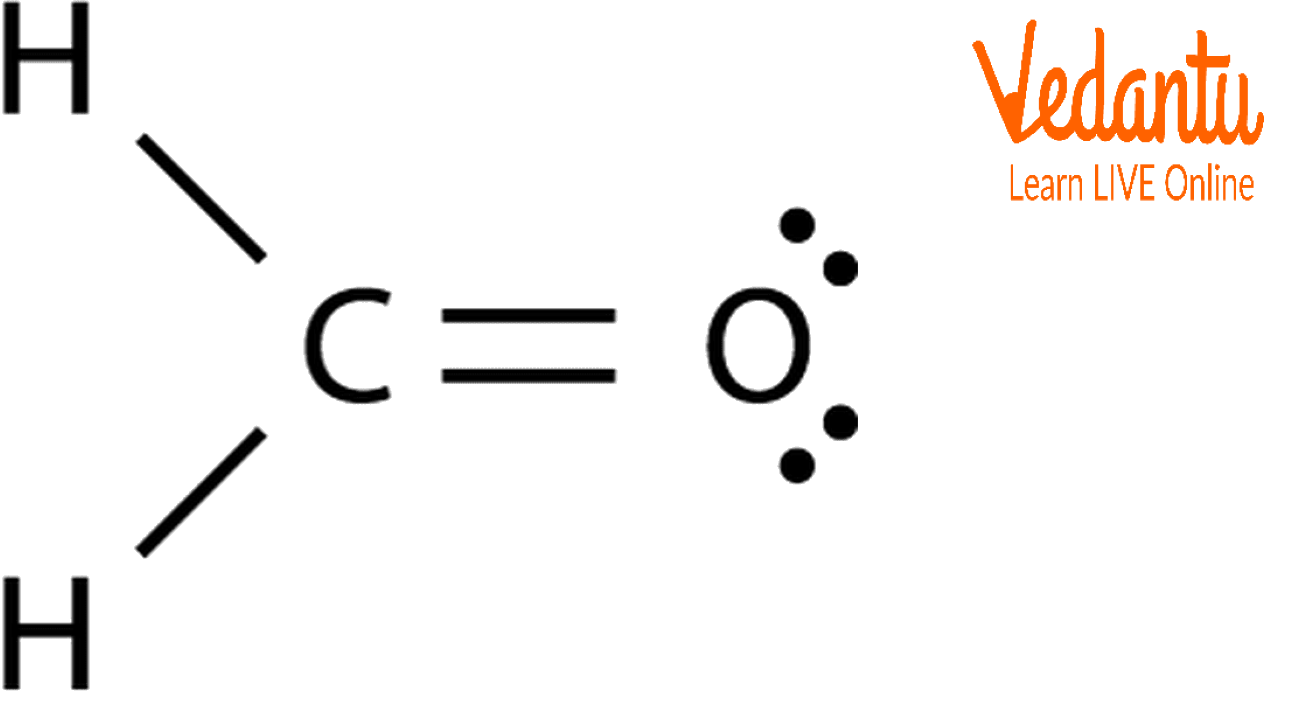
CH2O Polar or Nonpolar Learn Important Terms and Concepts

Draw the Structure of Ch2o Including Lone Pairs
Lewis Structure Of Ch2o

Draw the Structure of Ch2o Including Lone Pairs

Ch2o Lewis Structure And Vsepr Model

lewis structure of ch2o

CH2O Lewis Structure, Molecular Geometry, and Hybridization
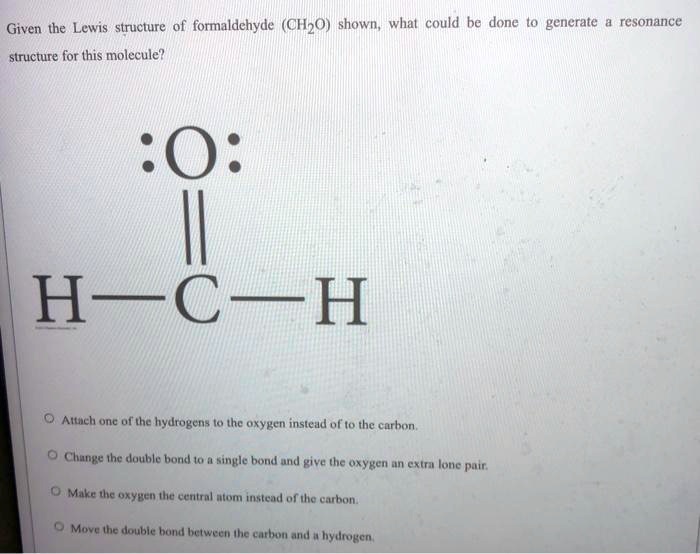
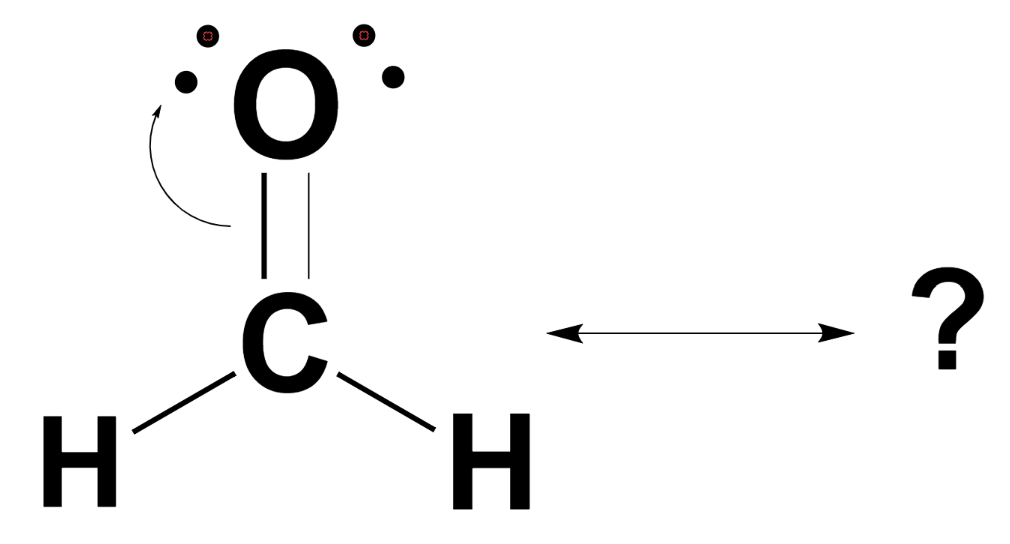
SOLVED Given the Lewis structure of formaldehyde (CH2O) shown; whal

CH2O Lewis Structure How to Draw the Dot Structure for CH2O YouTube

CH2O Molecular Geometry / Shape and Bond Angles YouTube
Web Draw The Structure Of Ch2O Including Lone Pairs.
Lewis Structure Is A Pictorial Representation Of The Atoms In The Molecules, Their Bonds, And Lone Pairs Of Electrons.
Draw The Lewis Structure For Formaldehyde, Ch, O.
Web How To Draw The Lewis Dot Structure For Ch2O:
Related Post: