Draw The Lewis Structure For The Formaldehyde Ch2O Molecule
Draw The Lewis Structure For The Formaldehyde Ch2O Molecule - Start with the valence electrons. But we have two hydrogens, so let's multiply that by 2. It is twelve as two are coming from the two hydrogen atoms, four from the carbon atom, and six from the oxygen atom. Web formaldehyde is a toxic organic molecule with molecular formula ch 2 o. 215k views 10 years ago. Web let's do the lewis structure for ch2o, methanal or formaldehyde. Based on their placement in the periodic table: Be sure to include all resonance structures that satisfy the octet rule. There are following specifications in the lewis structure of cl 2 o 5. 4 plus 2 plus 6 equals 12 total valence electrons to work with. There is one carbon, two hydrogen and one oxygen atoms participate in the formation of formaldehyde. Hydrogen has 1 valence electrons. Lewis structures show all of the valence electrons in an atom or molecule. Find the total number of valence electrons. Formaldehyde has a carbon atom double bonded to the oxygen and then single bonded to each of the hydrogens.more. Web let's do the lewis structure for ch2o, methanal or formaldehyde. Carbon atom is located as the center atom in hcho. Be sure to include all resonance structures that satisfy the octet rule. Draw the lewis structure for the formaldehyde (ch2o) molecule. There is a double bond between the carbon atom and the oxygen atom (o), which has two lone. Identify the total number of valence electrons for each atom. Total valence electrons = 1 carbon (4) + 2 hydrogen (2 x 1) + 1. Draw the lewis structure for the formaldehyde (ch2o) molecule. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. (2) draw single bonds between bonded atoms. Web the lewis structure of ch2o is drawn as: Total valence electrons = 1 carbon (4) + 2 hydrogen (2 x 1) + 1. Carbon (c) is the least electronegative atom in the ch2o lewis structure and. Lewis structures show all of the valence electrons in an atom or molecule. 4 (c) + 2 (h) + 6 (o) = 12. Carbon has 4 valence electrons. There is a double bond between the carbon atom and the oxygen atom (o), which has two lone pairs of electrons. Web the lewis diagram for formaldehyde (ch2o) is as follows: Carbon (c) is the least electronegative atom in the ch2o lewis structure and. (1) find the number of valence electrons in the molecule. Draw the lewis structure for the formaldehyde (ch2o) molecule. Start with the valence electrons. A lewis structure is a way to show how atoms share electrons when they form a molecule. Based on their placement in the periodic table: I quickly take you through how to draw the lewis structure of ch2o (methanal or formaldehyde). There is a double bond between oxygen and carbon atom. 91k views 12 years ago every video. Web here are the steps you need to follow to draw the lewis structure of ch 2 o: This problem has been solved! This problem has been solved! In this diagram, the carbon atom (c) is in the center with hydrogen atoms (h) on either side. Start with the valence electrons. Hydrogen, in group 1, has 1 and oxygen, in group 6 or 16, has 6; (1) find the number of valence electrons in the molecule. Be sure to include all resonance structures that satisfy the octet rule. Web hey guys,in this video, we are going to learn about the lewis structure of ch2o. At first, it is important to identify the number of participating elements in the formation of this compound. Draw out the lewis structure and use it to determine the electron domain geometry (edg) and molecular geometry (mg). I also go over hybridization, shape,. Web. (1) find the number of valence electrons in the molecule. Determine the total number of valence electrons: Web draw the lewis structure for the formaldehyde (ch2o) molecule. Web total valence electrons available for drawing the lewis structure of ch 2 o = 4 + 1 (2) + 6 = 12 valence electrons. Web the lewis diagram of a molecule can. 4 (c) + 2 (h) + 6 (o) = 12. Looking at the periodic table, carbon has 4. Hydrogen has 1 valence electrons. Lewis structures show all of the valence electrons in an atom or molecule. Search for the total already available valence electrons in a single formaldehyde ch2o molecule: 91k views 12 years ago every video. Formaldehyde is the simplest aldehyde made of hydrogen, carbon and oxygen with the. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web total valence electrons available for drawing the lewis structure of ch 2 o = 4 + 1 (2) + 6 = 12 valence electrons. Be sure to include all resonance structures that satisfy the octet rule. Total valence electrons = 1 carbon (4) + 2 hydrogen (2 x 1) + 1. There is one carbon, two hydrogen and one oxygen atoms participate in the formation of formaldehyde. Web steps of drawing. I quickly take you through how to draw the lewis structure of ch2o (methanal or formaldehyde). Find the total number of valence electrons. Draw out the lewis structure and use it to determine the electron domain geometry (edg) and molecular geometry (mg).
CH2O Lewis Structure, Molecular Geometry, and Hybridization
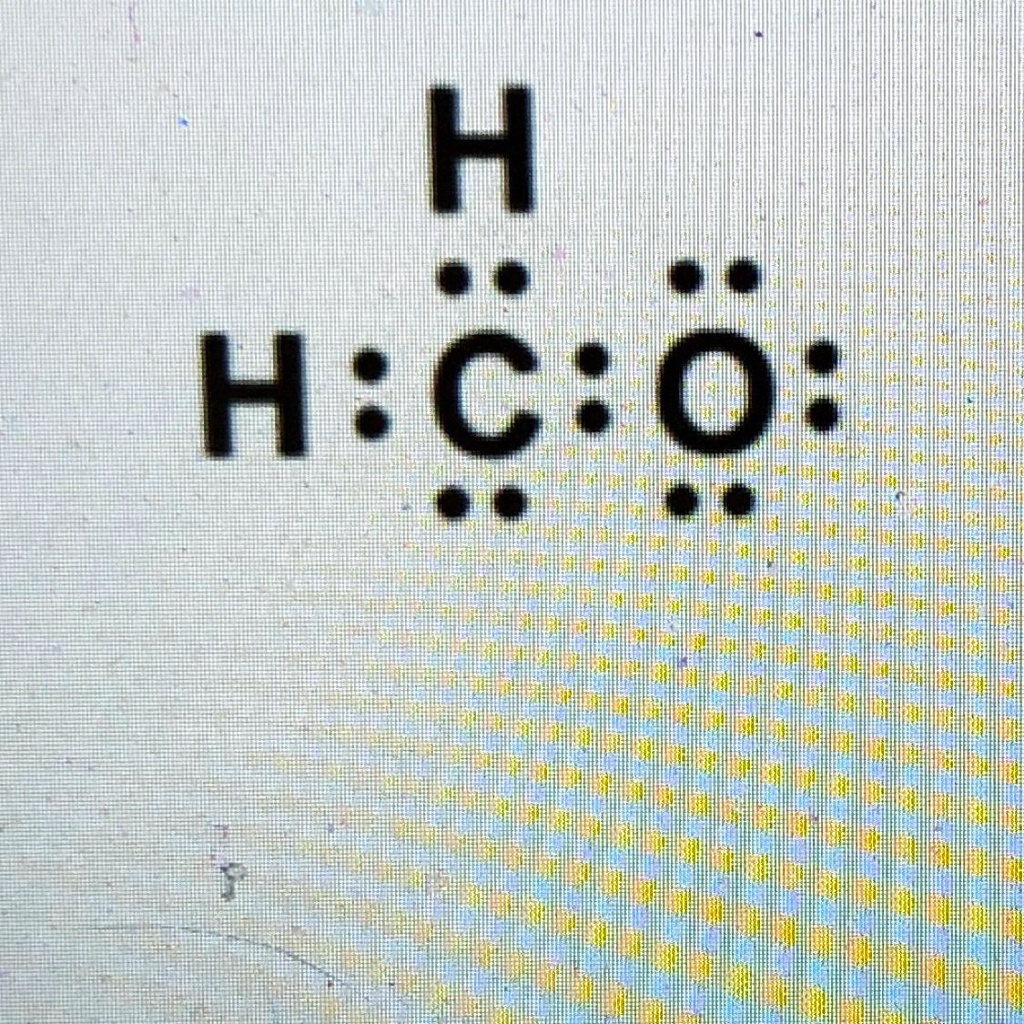
SOLVED 'Examine the Lewis structure below. If it is a correct

Formaldehyde Ch2o Molecule Chemical Structure Stock Illustration

How to Draw the Lewis Dot Structure for CH2O (Formaldehyde) YouTube

How to Draw the Lewis Dot Structure for CH2O Formaldehyde YouTube
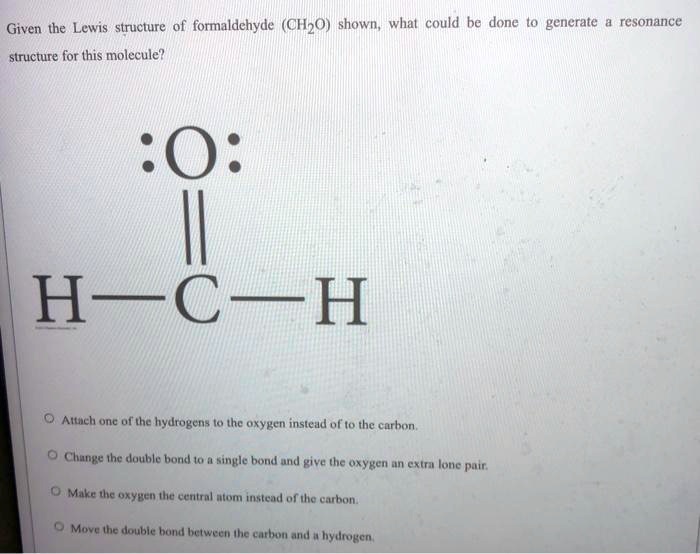
SOLVED Given the Lewis structure of formaldehyde (CH2O) shown; whal

CH2O Lewis Structure Lewis Dot Structure for CH2O Methanal or

CH2O Lewis Structure, Molecular Geometry, and Hybridization
:max_bytes(150000):strip_icc()/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)
How to Draw a Lewis Structure

Formaldehyde (CH2O) molecule, chemical structure. Atoms are represented
It Is A Chemical Formula For Methanol Or What Is Also Commonly Referred To A.
Carbon Has 4 Valence Electrons.
Web To Draw Lewis Structures For Molecules And Polyatomic Ions With One Central Atom.
I Also Go Over Hybridization, Shape,.
Related Post: