Draw The Lewis Structure For Of2
Draw The Lewis Structure For Of2 - Lewis structures show all of the valence electrons in an atom or molecule. A lewis structure is a way to show how atoms share electrons when they form a molecule. The ocl− o c l − ion. Web how to draw lewis structure for of2 oxygen difluoridelewis structure: Draw lewis structures for molecules. Determine the total number of valence (outer shell) electrons in the molecule or ion. Web to draw the of2 structure, place the central oxygen (o) atom and connect it to a fluorine (f) atom using a single bond, then distribute the remaining valence electrons. Remember that the least electronegative atoms go on the outside of the dot structure. #2 mark lone pairs on the atoms. Watch the video and see if you missed any steps or information. Web to draw lewis structures for molecules and polyatomic ions with one central atom. #3 calculate and mark formal charges on the atoms, if required. #1 first draw a rough sketch. Web how to draw lewis structure for of2? First step is to find the no. Draw lewis structures for molecules. In order to draw the lewis structure of of2, first of all you have to find the total number of valence electrons present in the of2 molecule. Web to draw the of2 structure, place the central oxygen (o) atom and connect it to a fluorine (f) atom using a single bond, then distribute the remaining. #1 first draw a rough sketch. Try structures similar to of 2 (like c h 4) for more practice. #1 first draw a rough sketch. First, determine the total number of valence electrons. The valence electrons are the electrons in the. The h2o h 2 o molecule. Determine the total number of valence electrons by adding up the valence electrons of all the atoms in the molecule. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. [ select] b) how many lone pairs of electrons are present on the central atom in the lewis structure?. It has a linear molecular geometry and sp3 hybridization. Oxygen belongs to group 16th and fluorine belongs to group 17th of the periodic table. We also use lewis symbols to indicate the formation of covalent bonds, which are shown in lewis structures, drawings that describe the bonding in molecules and polyatomic ions. (valence electrons are the number of electrons present. #1 first draw a rough sketch. Draw the correct lewis structure of of2 and then use it to answer the questions below. Find the total number of valence electrons. Web how to draw lewis structure for of2? Calculate the total number of valence electrons. For the of 2 lewis structure you'll need to decide which element goes in the center of the structure (it's fluorine). Web how to draw lewis structure for of2 oxygen difluoridelewis structure: Web drawing the lewis structure of of2. #1 first draw a rough sketch. #3 calculate and mark formal charges on the atoms, if required. Web it is helpful if you: #1 first draw a rough sketch. Oxygen belongs to group 16th and fluorine belongs to group 17th of the periodic table. The ocl− o c l − ion. Draw lewis structures for molecules. #2 mark lone pairs on the atoms. [ select] b) how many lone pairs of electrons are present on the central atom in the lewis structure? Count total valence electrons present in of2 molecule. Watch the video and see if you missed any steps or information. 13k views 1 year ago. Draw lewis structures for molecules. Web in the lewis structure of of2, both fluorine atoms share a single bond with the oxygen. We also use lewis symbols to indicate the formation of covalent bonds, which are shown in lewis structures, drawings that describe the bonding in molecules and polyatomic ions. Web it is helpful if you: 13k views 1 year. The h2o h 2 o molecule. Draw the correct lewis structure of of2 and then use it to answer the questions below. Find the total valence electrons in of2 molecule. First step is to find the no. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. Web now, we will discuss the steps to form the lewis structure of of2. Find the total number of valence electrons. It has a linear molecular geometry and sp3 hybridization. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. [ select ] c) what is the molecular geometry of of2? Count total valence electrons present in of2 molecule. Watch the video and see if you missed any steps or information. Oxygen has six valence electrons, while each fluorine atom has seven valence electrons, so of2 has a total of 6 + 2 (7) = 20 valence electrons. Draw lewis structures for molecules. The valence electrons are the electrons in the. Oxygen belongs to group 16, the chalcogen family, and has a valency of 6.[Solved] draw the lewis dot structure for the following molecule/ion
[Solved] Draw a Lewis structure for each molecular compound a. OF2 b
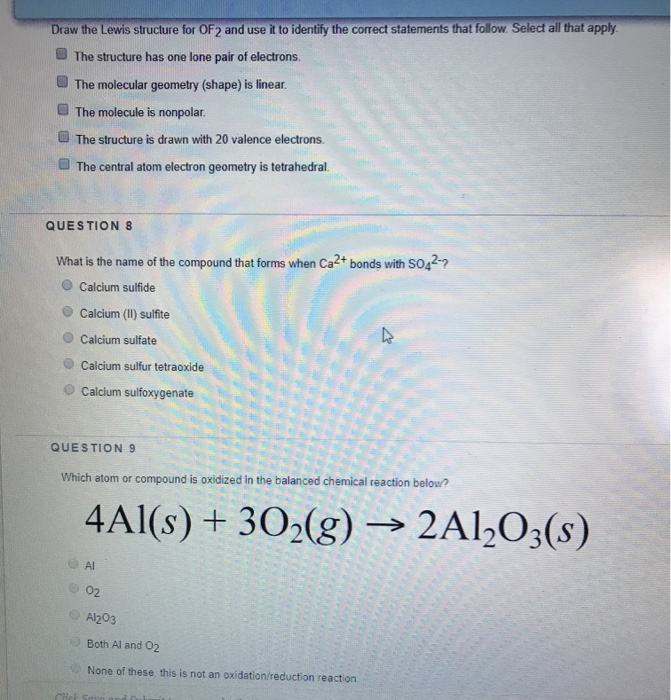
Solved Draw the Lewis structure for OF2 and use it to

Oxygen Difluoride Lewis Structure
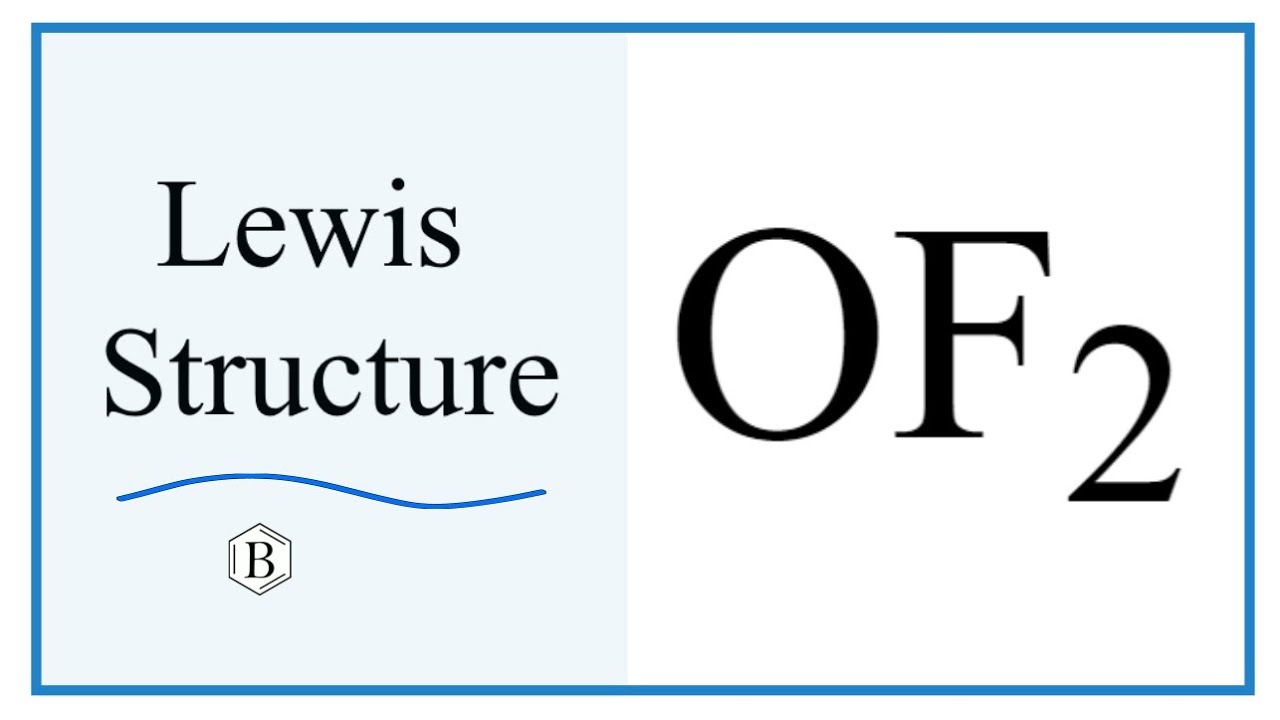
OF2 lewis structure, molecular geometry, hybridization and bond angle
Lewis Dot Structure for OF2 (Oxygen difluroide) YouTube
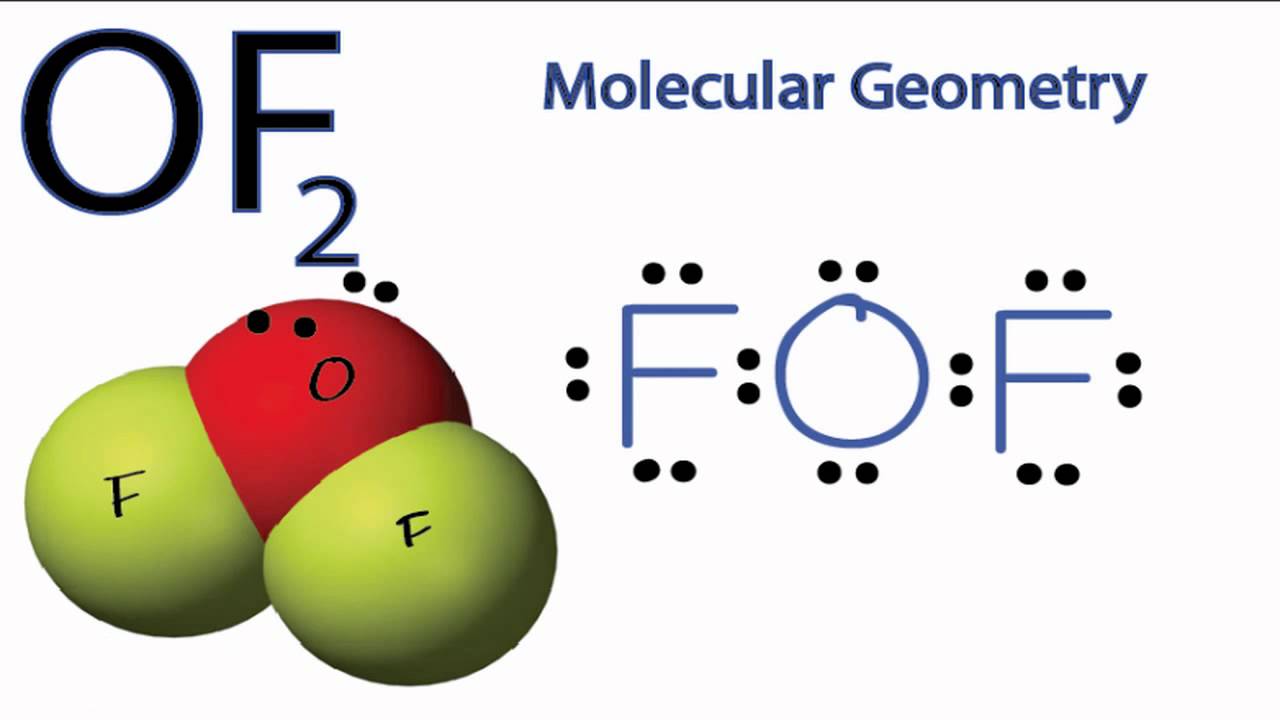
OF2 Molecular Geometry (note precise bond angle is 103.1) YouTube

OF2 Lewis Structure How to Draw the Lewis Structure for OF2 YouTube
Solved Draw the correct Lewis structure of OF2 and then use

Drawing the Lewis Structure of OF2 (Oxygen Difluride) YouTube
First, Determine The Total Number Of Valence Electrons.
Oxygen Brings 6 Electrons, Each Fluorine Brings 7 Electrons,.
#3 Calculate And Mark Formal Charges On The Atoms, If Required.
Of Valence Electrons Present In Of2 Molecule.
Related Post:
