Draw The Lewis Structure For Co2
Draw The Lewis Structure For Co2 - The basics of lewis structures. Web we can draw the lewis structure of any covalent molecule by following the six steps discussed earlier. Web the lewis structure provides a visual representation of the electron distribution in co2, highlighting the importance of valence electrons in determining how atoms interact and form bonds. Calculate the number of valence electrons: Each oxygen atom has two lone pairs and carbon atom does not have a lone pair. Send feedback | visit wolfram|alpha. For the carbon dioxide structure we use the periodic table to find the total number of. Web draw lewis structures depicting the bonding in simple molecules. On the periodic table, carbon is in group 4, or 14 sometimes; Draw a skeleton joining the atoms by single bonds. Calculate the number of valence electrons: 8 + (6 × × 7) = 50; For the co2 structure use the periodic table to find the total number of valence electrons for the co2. Molecular geometry of co 2; Web draw lewis structures depicting the bonding in simple molecules. Web lewis dot structure is a pictorial representation of the arrangement of the valence shell electrons in the molecule. Drawing lines represent the bonds formed in the molecule. Web so lewis structure generally gives us an idea about the nature of bonding and octet fulfillment of the atoms. Web draw lewis structures depicting the bonding in simple molecules. In order. Web this chemistry video explains how to draw the lewis structure of co2 also known as carbon dioxide. Web steps of drawing the lewis structure of co 2 are explained in detail in this tutorial. Drawing lines represent the bonds formed in the molecule. Also, there are no charges in oxygen atoms and carbon atom. The basics of lewis structures. Molecular geometry of co 2; Draw a trial structure by putting electron pairs around every atom until each gets an octet. Send feedback | visit wolfram|alpha. Calculate the number of valence electrons: Here’s how to approach this question. Web the lewis structure provides a visual representation of the electron distribution in co2, highlighting the importance of valence electrons in determining how atoms interact and form bonds. Find more chemistry widgets in wolfram|alpha. I also go over hybridization, shape and bond angles. For example, in co2, carbon needs 6 electrons to fulfill the octet, whereas oxygen needs only 2. It also discusses the bond angle, molecular geometry, and hybridization of the co2. Web lewis dot structure is a pictorial representation of the arrangement of the valence shell electrons in the molecule. In order to draw the lewis structure of co2, first of all you have to find the total number of valence electrons present in the co2 molecule. But. In order to draw the lewis structure of co2, first of all you have to find the total number of valence electrons present in the co2 molecule. Drawing lines represent the bonds formed in the molecule. Decide which is the central atom in the structure. I also go over hybridization, shape and bond angles. But we have two of them. Molecular geometry of co 2; Web 6 steps to draw the lewis structure of co2 step #1: Web we can draw the lewis structure of any covalent molecule by following the six steps discussed earlier. Then, minimize these formal charges by converting the lone pairs of atoms to chemical bonds. Co 2 lewis structure and shape. For the co2 structure use the periodic table to find the total number of valence electrons for the co2. In the lewis structure of co 2, you can see there are two double bonds around carbon atom. Web this widget gets the lewis structure of chemical compounds. Web i quickly take you through how to draw the lewis structure of. 8 + (6 × × 7) = 50; Web how to draw lewis structure for co 2; Then, minimize these formal charges by converting the lone pairs of atoms to chemical bonds. Web we're going to do the lewis structure for co2, carbon dioxide. Draw a trial structure by putting electron pairs around every atom until each gets an octet. Calculate the total number of valence electrons. Each oxygen atom has two lone pairs and carbon atom does not have a lone pair. Step method to draw the lewis structure of co 2. In the lewis structure of co 2, you can see there are two double bonds around carbon atom. Molecular geometry of co 2; Co 2 lewis structure and shape. Here, the given molecule is co2 (carbon dioxide). In this case, we can condense the last few steps, since not all of them apply. Web for very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. These valence electrons are represented by drawing dots around the individual atoms, hence the lewis dot structure. A lewis structure is a way to show how atoms share electrons when they form a molecule. Draw a skeleton joining the atoms by single bonds. Web lewis dot structure is a pictorial representation of the arrangement of the valence shell electrons in the molecule. Web we're going to do the lewis structure for co2, carbon dioxide. Web to draw a co 2 lewis structure, begin by sketching out a rough structure of the molecule and indicating the locations of any lone pairs on the atoms. Here’s how to approach this question.
Draw The Lewis Structure For CO2

Draw Lewis Structure

CO2 Lewis Structure How to Draw the Dot Structure for Carbon Dioxide
:max_bytes(150000):strip_icc()/CO2LewisStructure-591c94063df78cf5fadfde77.png)
CO2 Molecule Lewis Structure
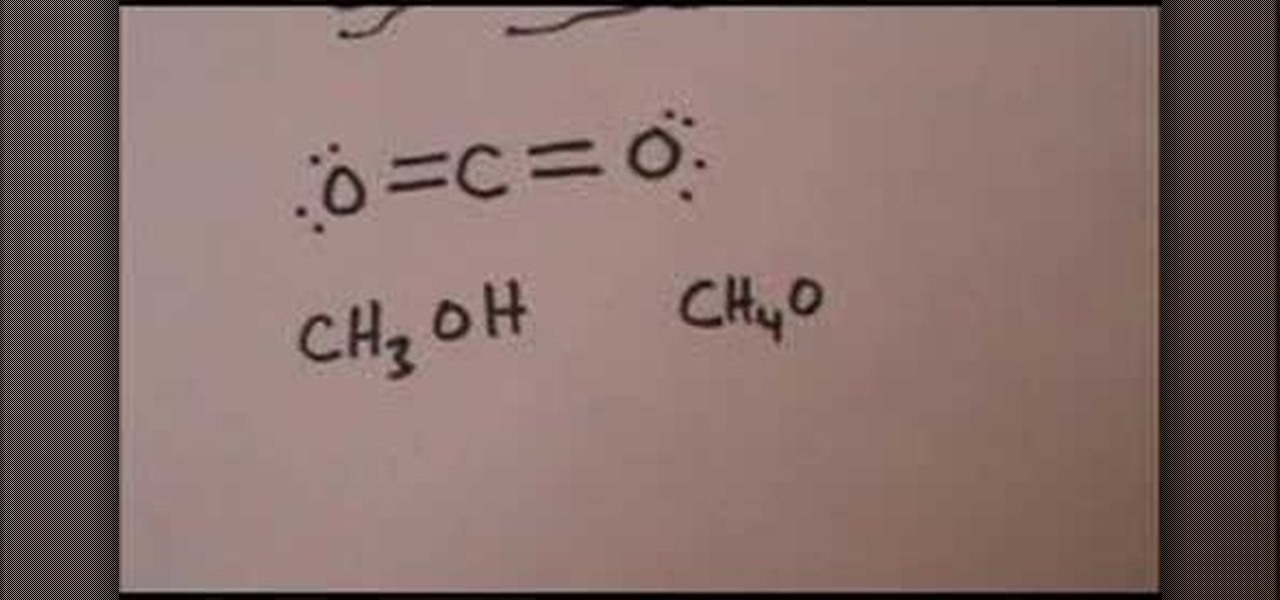
Draw The Lewis Structure For CO2

Draw The Lewis Structure For CO2

Lewis Structure For Co23 Drawing Easy

Best Describes the Shape of the Carbon Dioxide Co2 Molecule

What is the Lewis Dot structure for CO2 (Carbon dioxide)?
CO2 Lewis Structure How to Draw the Dot Structure for Carbon Dioxide
Web We Can Draw The Lewis Structure Of Any Covalent Molecule By Following The Six Steps Discussed Earlier.
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Web Steps Of Drawing The Lewis Structure Of Co 2 Are Explained In Detail In This Tutorial.
8 + (2 × × 7) = 22 Xef 6:
Related Post: