Draw Sodium Atom
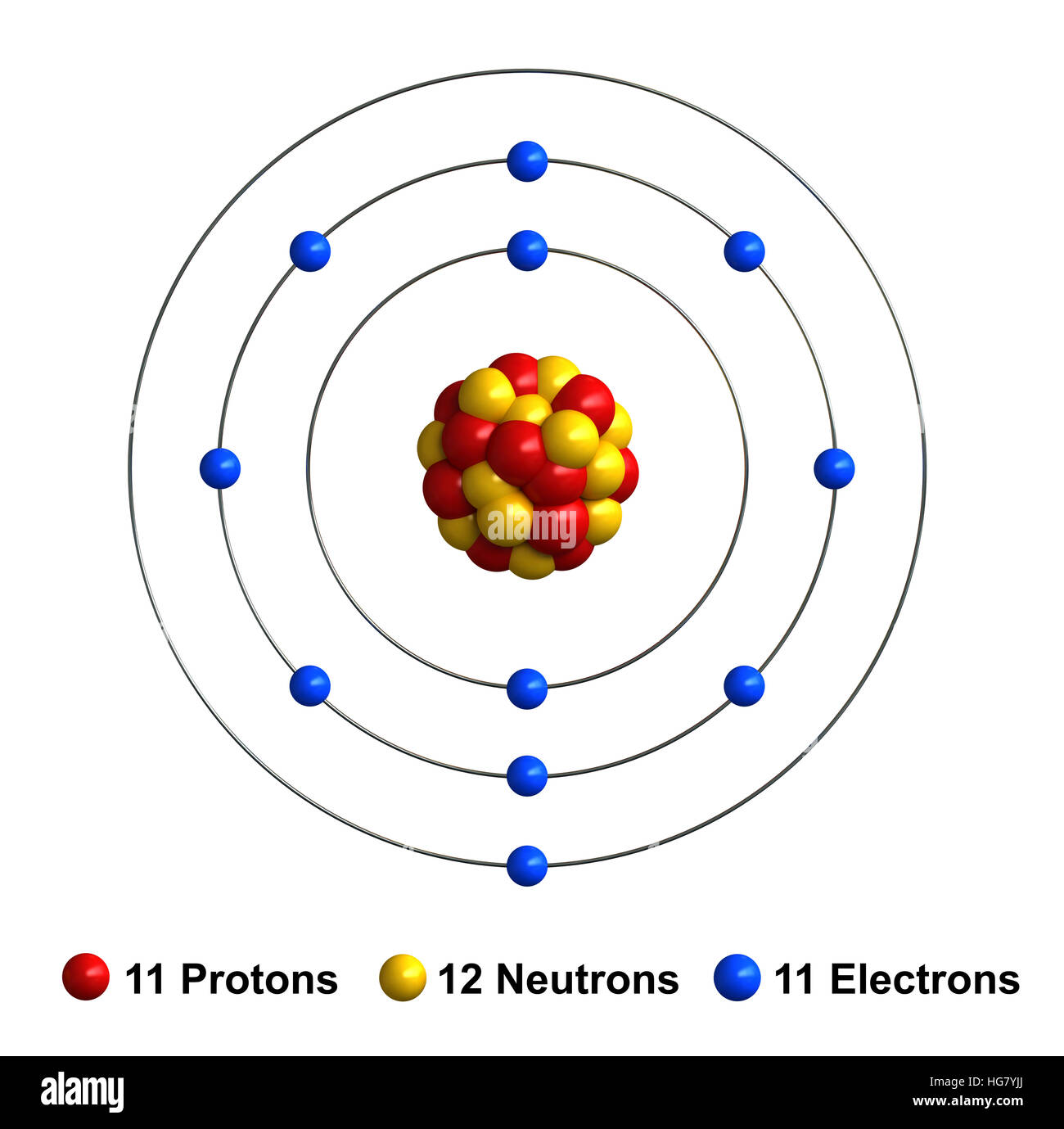
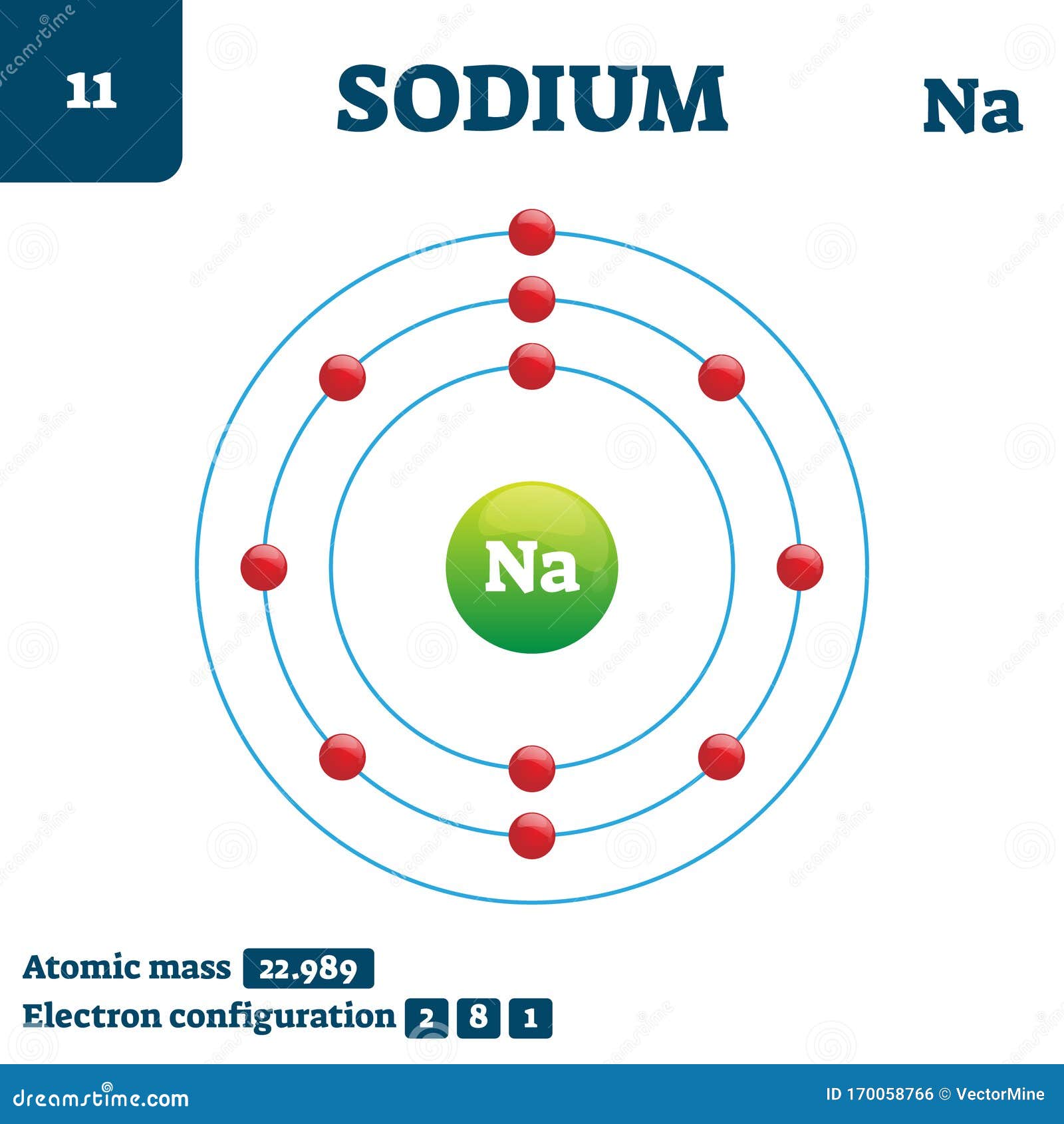
Draw Sodium Atom - Add lone pair electrons to each oxygen atom. A sodium cation (na⁺) has one fewer electron, resulting in a +1 overall charge. I show you where sodium is on the periodic table and how to determine how many. Neutral sodium atom has 11 electrons whereas sodium ion ( n a +) has 10 electrons. By the end of this section, you will be able to: The darker the region, the more likely electrons are to be found there. Draw lewis structures depicting the bonding in simple molecules. Although we have discussed the general arrangement of subatomic particles in atoms, we have said little about how electrons occupy the space about the nucleus. For a neutral atom, the number of protons equals the number of electrons, making the overall charge zero. For the na+ structure use the periodic table to find the total number of valence electrons for. The sodium atom (na) is commonly used for examples and practice problems in chemistry. A sodium atom can be drawn with a nucleus containing 11 protons and 12 neutrons, surrounded by 11 electrons in respective energy levels. Describe how electrons are grouped within atoms. Web the bohr model of sodium (na) is drawn with three electron shells, the first shell. Write lewis symbols for neutral atoms and ions. A picture of a hydrogen atom can be found here. For a neutral atom, the number of protons equals the number of electrons, making the overall charge zero. Draw lewis structures depicting the bonding in simple molecules. Connect the sodium atom to a carbon (c) atom with a double bond. A sodium atom has 1 electron in its outer shell. Web the bohr model of sodium (na) is drawn with three electron shells, the first shell contains 2 electrons, the second shell contains 8 electrons and the third shell contains 1 electron. I show you where sodium is on the periodic table and how to determine how many. We draw. Web sodium is used as a heat exchanger in some nuclear reactors, and as a reagent in the chemicals industry. So, if you want to draw atomic structure for sodium first of all you should know the atomic number for sodium. Write lewis symbols for neutral atoms and ions. I show you where sodium is on the periodic table and. It is an alkali metal and a member of the group 1 elements in the periodic table. Electron shells niels bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. 62k views 10 years ago. For a neutral atom, the number of protons equals the number of electrons,. Neutral sodium atom has 11 electrons whereas sodium ion ( n a +) has 10 electrons. Connect the sodium atom to a carbon (c) atom with a double bond. So, if you want to draw atomic structure for sodium first of all you should know the atomic number for sodium. Web 1 fm = 10 − 15 m. The first. 140k views 5 years ago. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. A sodium cation (na⁺) has one fewer electron, resulting in a +1 overall charge. In contrast, chlorine and sodium have seven and one electrons in their outer shells, respectively. The first two shells should accommodate 2. 140k views 5 years ago. Web join teryn and me in this educational art lesson, where we’ll teach you how to draw a basic atom. Draw lewis structures depicting the bonding in simple molecules. Describe how electrons are grouped within atoms. 62k views 10 years ago. A sodium atom has 1 electron in its outer shell. In contrast, chlorine and sodium have seven and one electrons in their outer shells, respectively. Describe how electrons are grouped within atoms. Web draw the bohr diagram of an atom with 18 electrons or fewer. Sodium is neutral and its atomic number is 11, hence, the number of protons and. For a neutral atom, the number of protons equals the number of electrons, making the overall charge zero. Describe how electrons are grouped within atoms. Web as shown, helium has a complete outer electron shell, with two electrons filling its first and only shell. Electron shells niels bohr proposed an early model of the atom as a central nucleus containing. So, if you want to draw atomic structure for sodium first of all you should know the atomic number for sodium. A sodium cation (na⁺) has one fewer electron, resulting in a +1 overall charge. We draw atomic structures for any element with the help of atomic number they have. Web 1 fm = 10 − 15 m. Web draw the bohr diagram of an atom with 18 electrons or fewer. Place a sodium (na) atom in the center of the grid. Web this video shows how to draw the orbital diagram of sodium (na). 61k views 4 years ago. By the end of this section, you will be able to: Web sodium is used as a heat exchanger in some nuclear reactors, and as a reagent in the chemicals industry. Sodium is neutral and its atomic number is 11, hence, the number of protons and electrons available for its bohr diagram is also 11. Web in contrast, chlorine has only seven electrons in its outermost shell, while sodium has just one. I show you where sodium is on the periodic table and how to determine how many. For a neutral atom, the number of protons equals the number of electrons, making the overall charge zero. Draw lewis structures depicting the bonding in simple molecules. Web as shown, helium has a complete outer electron shell, with two electrons filling its first and only shell.
FileElectron shell 011 sodium.png Wikimedia Commons
:max_bytes(150000):strip_icc()/sodiumatom-58b602715f9b5860464c7a22.jpg)
Atom Diagrams Electron Configurations of the Elements

Premium Vector Sodium atom bohr model
3d render of atom structure of sodium isolated over white background
Sodium atom polizworth

sodium electron configuration Newton Desk

The sodium atom YouTube
Chemistry 2.Draw the atomic structure of a sodium atom and a sodium

Atom sodium Royalty Free Vector Image VectorStock

How To Draw A Sodium Ion Image to u
The Most Common Compound Of Sodium Is Sodium Chloride (Common Salt).
Perfect For Young Learners And Aspiring Artists, This Simple Tutorial Uses Art For Kids Hub Markers And Marker Paper.
The Nucleus Is Shown As A Tiny Clump Of Red Protons And Purple Neutrons In The Center Of The Atom.
Atomic Number Of Sodium Is 11.
Related Post: