Draw An Atom Of Oxygen
Draw An Atom Of Oxygen - Oxygen is the eighth element with a total of 8 electrons. Give today and help us reach more students. Energy p 4 enter orbital label continue. Web a diagram of an oxygen atom. 6 valence electrons/atom × 1 atom = 6 + f: I show you where oxygen is on the periodic table and how to determine how many valence electrons it has. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on. An ion of an atom is one in which the number of protons and. Members of a group typically have similar properties and electron configurations in their outer shell. I even had them draw a few atoms with the protons and neutrons in the center and the electrons in shells. A lewis structure is a way to show how atoms share electrons when they form a molecule. The electron dot diagram for an element shows the valence electrons for the element. 7 valence electrons/atom × 2 atoms = 14 ¯ = 20 valence electrons As seen in the above figure, we know that the atomic number of oxygen atoms is. Therefore, the number of protons in the oxygen atom is 8. Individual atoms are extremely small. To draw lewis structures for molecules and polyatomic ions with one central atom. Steps of drawing lewis structure of o 2 molecule. Remember, a neutral atom contains the same number of protons and electrons. The phet website does not support your browser. A rule stating that atoms lose, gain, or share electrons in order to have a full valence shell of 8 electrons. Oxygen is in group 16/via, so it has six valence electrons. There are several interesting steps in drawing oxygen molecule's lewis structure. Lewis structures show all of the valence electrons in. Define the atomic mass unit and average atomic mass. Oxygen is the eighth element with a total of 8 electrons. We’ll use a bohr diagram to visually represent where the electrons a. Steps of drawing lewis structure of o 2 molecule. I show you where oxygen is on the periodic table and how to determine how many valence electrons it. Groupa vertical column in the periodic table. A rule stating that atoms lose, gain, or share electrons in order to have a full valence shell of 8 electrons. The atomic number of each element increases by one, reading from left to right. This problem has been solved! Then play a game to test your ideas! We recommend using the latest version of chrome, firefox, safari, or edge. A rule stating that atoms lose, gain, or share electrons in order to have a full valence shell of 8 electrons. Remember, a neutral atom contains the same number of protons and electrons. Give today and help us reach more students. Web a diagram of an oxygen atom. We recommend using the latest version of chrome, firefox, safari, or edge. We’ll use a bohr diagram to visually represent where the electrons a. It would take about fifty million atoms in a row to make a line that is 1 cm long. Write and interpret symbols that depict the atomic number, mass number, and charge of an atom or. Web our mission is to improve educational access and learning for everyone. 6 valence electrons/atom × 1 atom = 6 + f: 6 valence electrons/atom × 1 atom = 6 + f: Web to begin with, let us calculate the number of protons for the oxygen atom, the atomic number of an element represents the number of protons present in. Individual atoms are extremely small. 7 valence electrons/atom × 2 atoms = 14 ¯ = 20 valence electrons of 2 o: Oxygen is the eighth element with a total of 8 electrons. There is a double bond between oxygen atoms and two lone pairs exist on each oxygen atom. Steps of drawing lewis structure of o 2 molecule. This problem has been solved! How to write the electron configuration for oxygen. Draw the electron configuration for a neutral atom of oxygen. Web the upper right side shows the number of electrons in a neutral atom. Write and interpret symbols that depict the atomic number, mass number, and charge of an atom or ion. Then place one dot at each side of the symbol. Give today and help us reach more students. The phet website does not support your browser. 7 valence electrons/atom × 2 atoms = 14 ¯ = 20 valence electrons Construct an atom according to the bohr model. 6 valence electrons/atom × 1 atom = 6 + f: Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Perioda horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. So that they’d have a bit of context, i went over the basic parts of an atom (protons, neutrons, and electrons) and made it clear that the name of the element is determined solely by the number of protons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web to begin with, let us calculate the number of protons for the oxygen atom, the atomic number of an element represents the number of protons present in that atom. I show you where oxygen is on the periodic table and how to determine how many valence electrons it has. State the modern atomic theory. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. This problem has been solved!
Drawing Atoms Montessori Muddle

Oxygen atom bohr model Royalty Free Vector Image
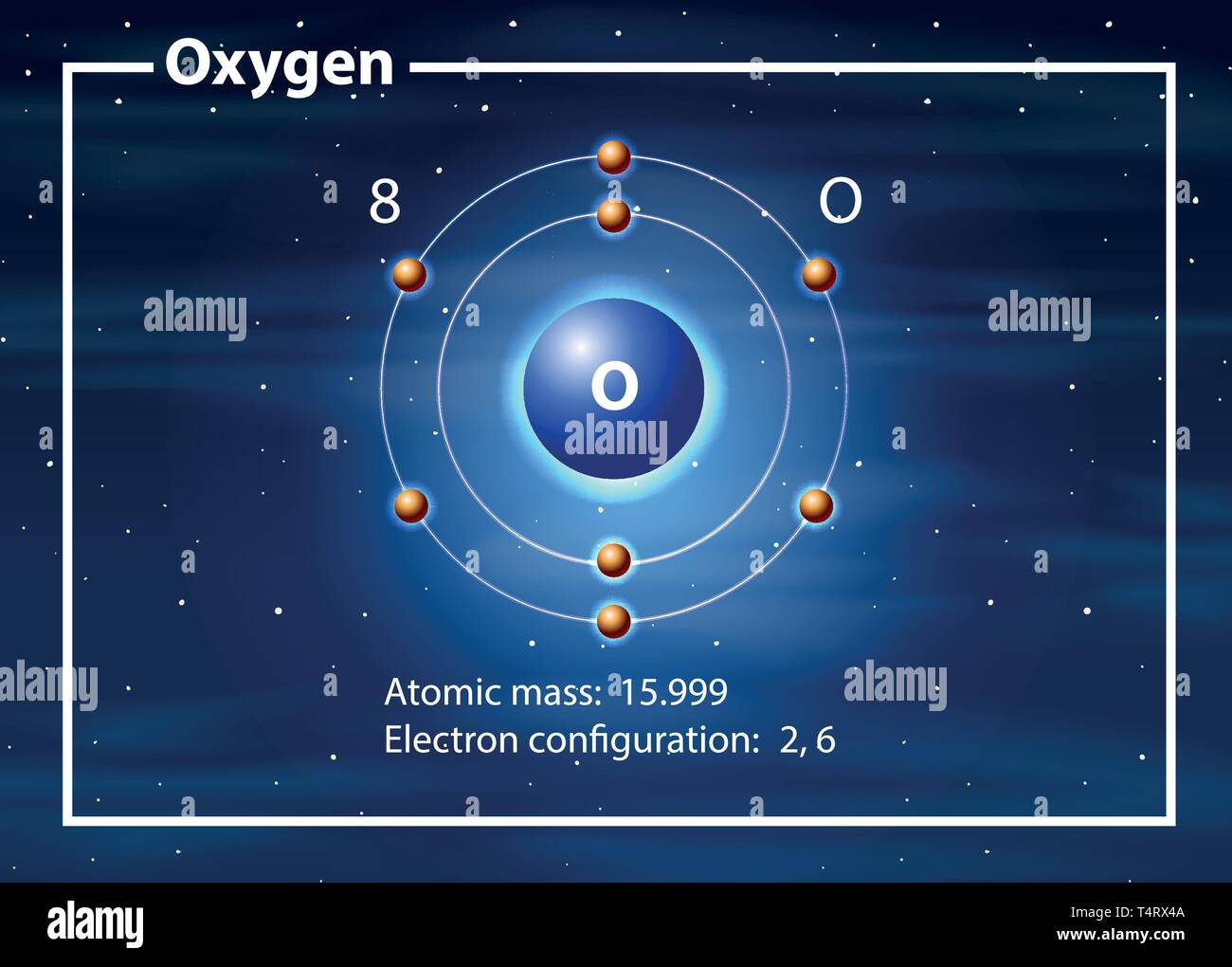
Oxygen atom diagram concept illustration Stock Vector Image & Art Alamy
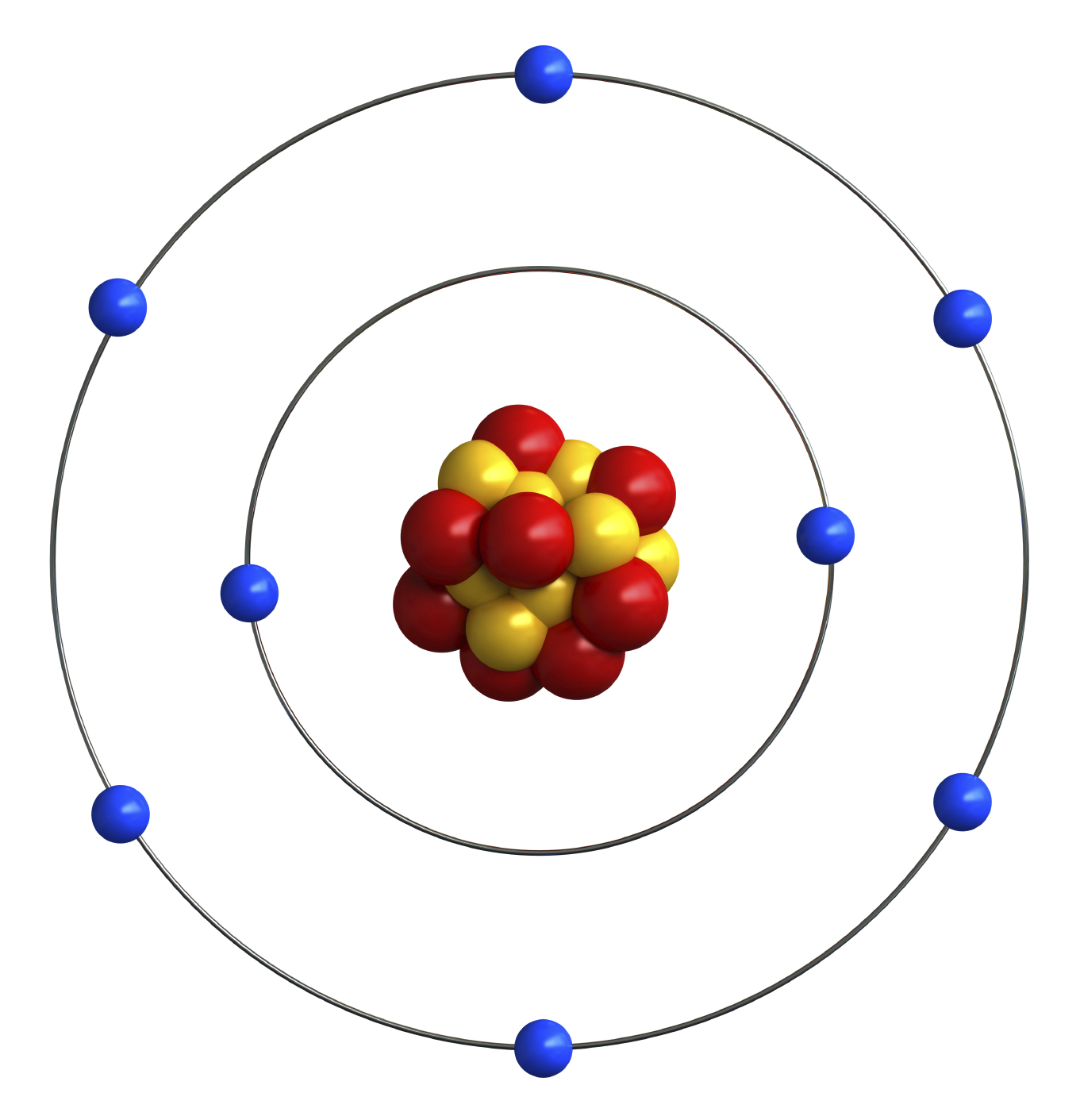
Forms of Energy ND Studies Energy Curriculum
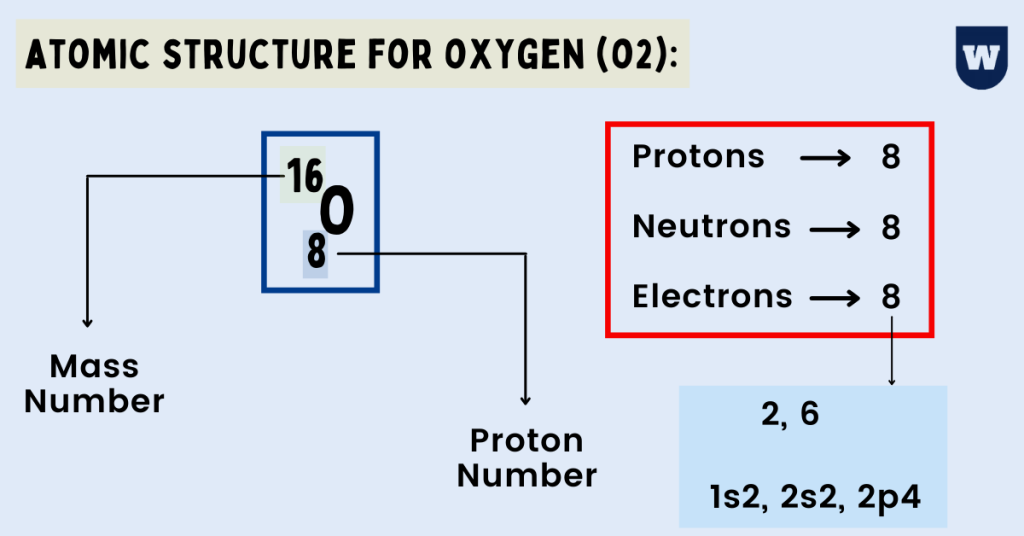
Atomic Structure for Oxygen (O2) Best Guide (With Diagrams)
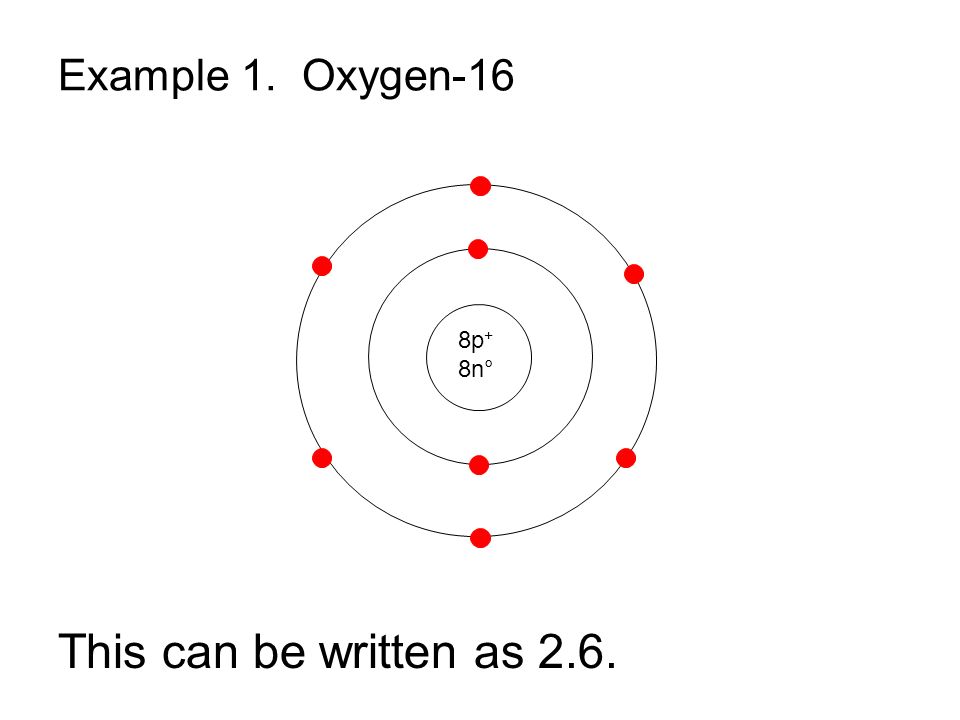
Bohr Model Drawing Of Oxygen at Explore collection

Molecular Structure of an Oxygen Atom Stock Vector Illustration of

Diagram representation of the element oxygen Vector Image

Oxygen Valence Electrons (O) Oxygen Valency & Electron Configuration
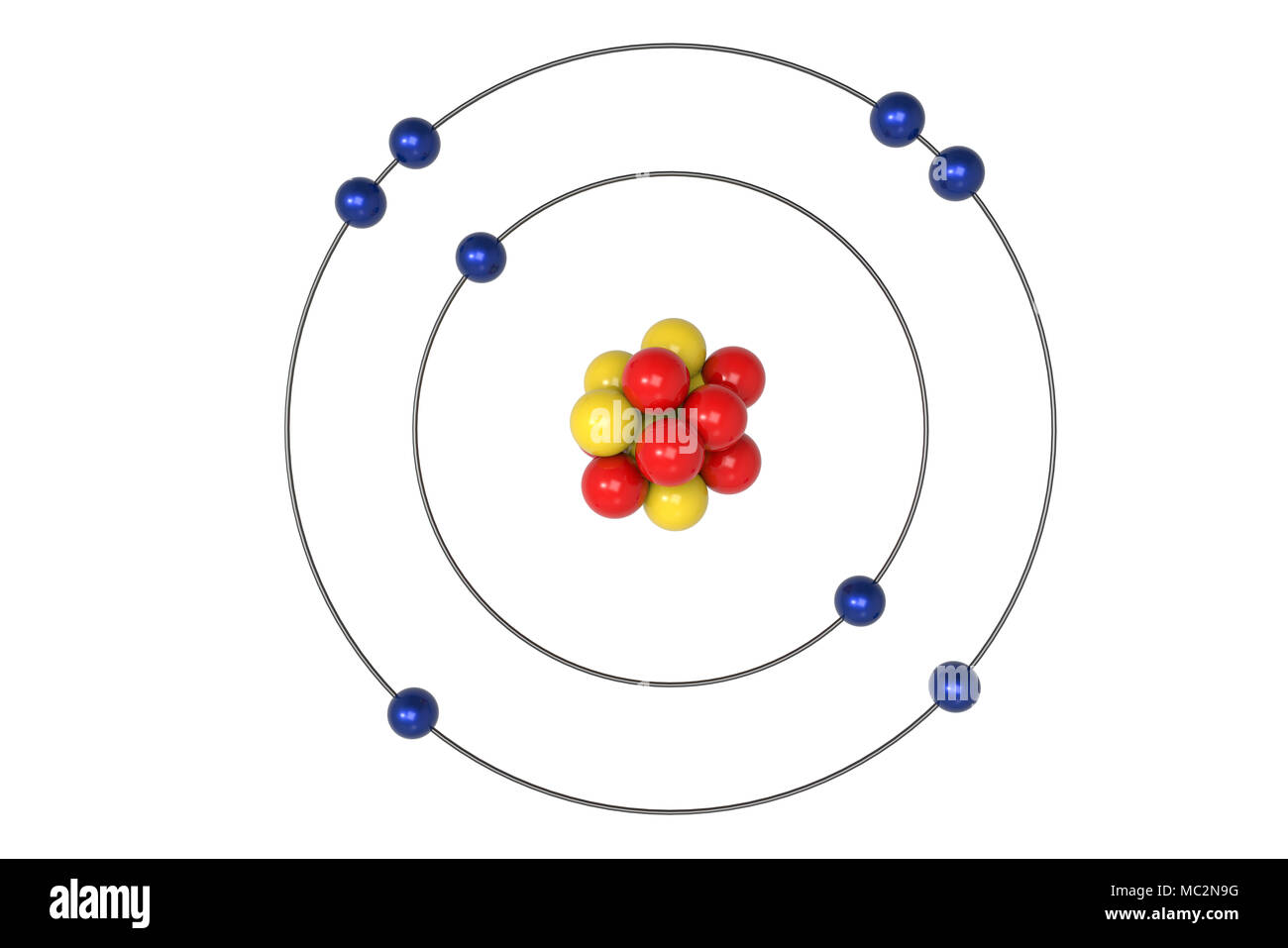
Oxygen Atom Bohr model with proton, neutron and electron. 3d
Web Our Mission Is To Improve Educational Access And Learning For Everyone.
The Isotope Is Defined By The Number Of Neutrons In An Atom, Which Might Be Equal To The Number Of Protons—Or Not.
Members Of A Group Typically Have Similar Properties And Electron Configurations In Their Outer Shell.
Define The Atomic Mass Unit And Average Atomic Mass.
Related Post: